Bath bombs are awesome balls of fizzy goodness, with some interesting science behind them! They were invented in 1989 by Mo Constantine, one of Lush’s founders. Bath bombs contain the chemical sodium bicarbonate, also known as baking soda, as their key ingredient.
This is the text version – scroll down for the video!
Some of you might remember that baking soda isn’t good for your skin because it’s a base, with a high pH. High pH (alkaline or basic) products disturb the skin’s acid mantle, which protects your living tissue from the environment, particularly bacteria, like acne-causing Propionibacterium acnes.
But don’t fret! The second key ingredient in a bath bomb is a solid acid, such as citric acid or tartaric acid (cream of tartar). This lowers the pH by reacting with the baking soda when water is added to the mixture. Unless the maker of the bath bombs has really messed up their proportions, the final pH should be reasonably neutral. Until the water dissolves the acid and baking soda and allows them to mix at a microscopic level, nothing happens.
Aside from neutralisation, the acid + base reaction with sodium carbonate also produces tiny bubbles of carbon dioxide gas, which is what causes the fizzing:
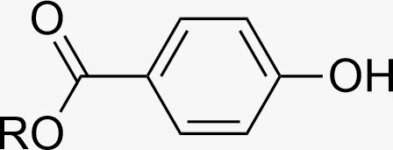
Citric acid + sodium bicarbonate → sodium citrate + water + carbon dioxide
C6H8O7(s) + 2NaHCO3(s) → Na2C6H6O7(aq) + 2H2O(l) + 2CO2(g)
This is almost the same reaction as the one commonly used in volcano science projects to create foaming “lava” (they usually use vinegar as the acid). Of course, in bath bombs, there’s also fragrance and colours and glitter – the fizzing helps the bath bomb disperse faster, and combined with heat from the hot water, spreads the scent faster and makes the whole bathroom smell amazing.
Bath bombs can slowly absorb water from the air, using up the acid and sodium carbonate and releasing carbon dioxide prematurely – this is why bath bombs get less fizzy as they get old! Make sure you keep your bath bombs in a dry place until you’re ready to use them.
Happy bathing!






Very cool, now I do t have to look it up! Thanks 🙂
*don’t
(Ugh)
Thanks for this article Michelle, I haven’t tried any of them yet, neither for bath not nails 🙂
Hi Michelle,
Great post.
I use products from Lush daily and a Lush bomb weekly and I never thought to find out exactly why they work as they do. I love both the golden wonder bath bomb and the snow angel bath melt, I wish they were a regular, not just a seasonal Lush product.
Regards
Aimee 🙂
http://www.laughinggalah.com/
I love Golden Wonder too! The stars and the blue colour and the random bobbing colours are magical.
I love making bath fizzles. Thanks for the article Michele!
According to this reaction: C6H8O7(s) + 3NaHCO3(s) → Na3C6H5O7(aq) + 3H2O(l) + 3CO2(g) sodium and citric acid should be added in molar ratio of 1,3:1 (252 g sodium: 192 g citric acid). So my question is: why the proportion in your recipe is 2:1?
Thanks for pointing that out, I didn’t catch it the first time! Full neutralisation isn’t really necessary for this reaction to go – a 2:1 ratio will just produce the C6H6O72- ion instead. It also has the advantage that it’s a weaker base, so the final pH will be closer to the skin’s. The equation would instead be
C6H8O7(s) + 2NaHCO3(s) → Na2C6H6O7(aq) + 2H2O(l) + 2CO2(g)
I’ve corrected it now 🙂
I was just going to mention this 🙂 Most bath bombs use a 2:1 ratio – some have even high citric acid if the goal is spinning/mega fizz. So the water will be acidic. Some bath bombs and other products have products that are buffers or water softeners so those will be neutralish.
awesome article! I am doing a science project on bath bombs for a 7th-grade science fair. Do you know the place of publication and publisher for this article/your website? Thank you!
Hi! I think I replied to you on IG already, but the publisher would just be Lab Muffin 🙂
HI, Michelle! how would I cite your website! I can’t seem to find you last name anywhere so it is kind of difficult to cite! Thank you!
Sorry! My last name is Wong 🙂