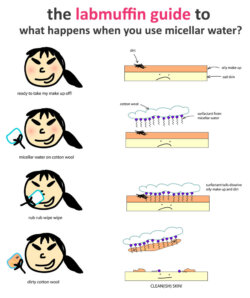
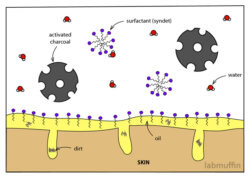
Fact-check: What is micellar water and how does it work? An Update
Over the past two years, my post on the chemistry of micellar water with dodgy photographed scrawlings has become one of the most popular, so I thought it was high time to update it with nicer drawings and finetune the explanation of the science. This also comes in video form – check it out here! There are tons of micellar …