If there’s one skincare ingredient that works, it’s retinol. Pretty much everyone agrees – whatever skin concern you have, it’s one of your best bets.
But is it all a scam?

Let’s dive into the science behind retinol, and the bigger question of why skincare science looks so different from what academic scientists are used to.
The video is here, keep scrolling for the written version…

Retinol doesn’t work?
Quite a few dermatologists and scientists don’t think retinol works. For example, this recent Science Vs podcast episode Skin Care: Is Anti-Aging a Scam? questioned the efficacy of retinol:
“…there is very little, if any, trustworthy evidence available to support the use of retinol-containing products to improve the appearance of aged skin.” (quoted from this paper)
Dr Natalia Spierings: “I think they are a total waste of time and money.”
I can see where they’re coming from. When I started looking at skincare during my medicinal chemistry PhD, I saw a huge difference between what scientists are taught is good evidence, and what I was seeing for skincare.
But after 12 years of studying skincare science, learning about the cosmetic industry and getting qualified as a cosmetic chemist, I feel like I can finally explain to you why skincare science looks so unsciencey, and how to find products that are more likely work.
(Some of you might be thinking, “she’s just defending this scam because she’s been sponsored by skincare companies before.” But a lot of non-sponsored scientists and dermatologists agree, and once I break it down, I think lots of things you’ve noticed about skincare will make a lot more sense, and you’ll find it easier to pick out evidence-based skincare products.)
What is retinol?
Retinol is part of the family of retinoids. These are ingredients based on vitamin A, and they’re the superstars of skincare – they’re effective for lots of skin concerns, like acne, pigment and wrinkles. But not all of them have the same level of evidence.
Some are drugs, which have been extensively studied, and that’s where a lot of the great reputation for retinoids comes from.
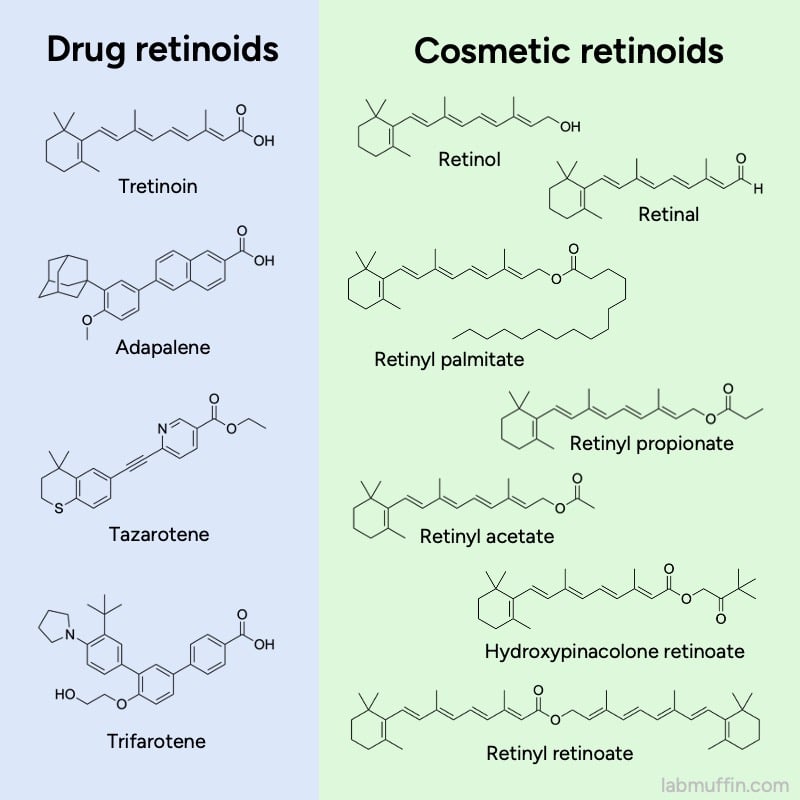
But what about retinol?
Clinical trial evidence for retinol
Peer-reviewed clinical trials are usually viewed as the ultimate test for the effectiveness of treatments. Clinical trials are where products are actually tested on people, and peer-reviewed means other scientists have looked over the study before it’s published.
Most peer-reviewed clinical trials are listed in the PubMed database. A cursory search turns up 39 clinical trials for retinol and skin, which is pretty low, and many of these aren’t actually on its use on skin.
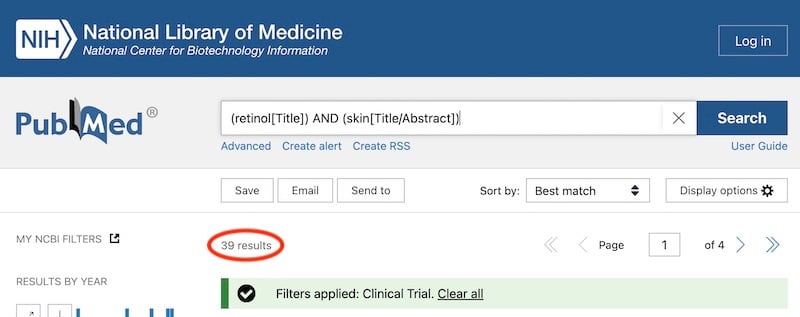
Here’s a typical retinol clinical trial (actually, one of the better ones out there!):

- It uses 40 subjects, which is low by medical study standards, but actually pretty high by skincare study standards
- It’s placebo-controlled, meaning the retinol product was compared to a cream without actives – again this is good study design, but most skincare studies don’t do this
- It’s double-blind, which means both the volunteers and people measuring skin changes don’t know if they’re applying the real product or the placebo, so they aren’t as biased
- But retinol is combined with lactose and glycolic acid, so this study won’t be able to tell you if it’s the retinol working or some other ingredient
- Most of the authors work for Johnson & Johnson, who make the product, so there’s potential conflict of interest
And this is just from the abstract – the study itself will probably have more issues!
So why does the clinical trial evidence for the “best” skincare ingredient look like this?
Cosmetic regulations
Well, a lot of this is the government’s fault.

Most countries draw a line between drugs and cosmetics.
Drugs are defined as products that can treat disease, or change the structure or function of skin.
For an ingredient to become a drug, it has to go through an approval process. This involves doing a whole bunch of studies, including on lots of people to show that it’s effective. The company then submits all this data to a regulatory agency, they make sure there’s enough evidence to say it works, and it becomes a drug.
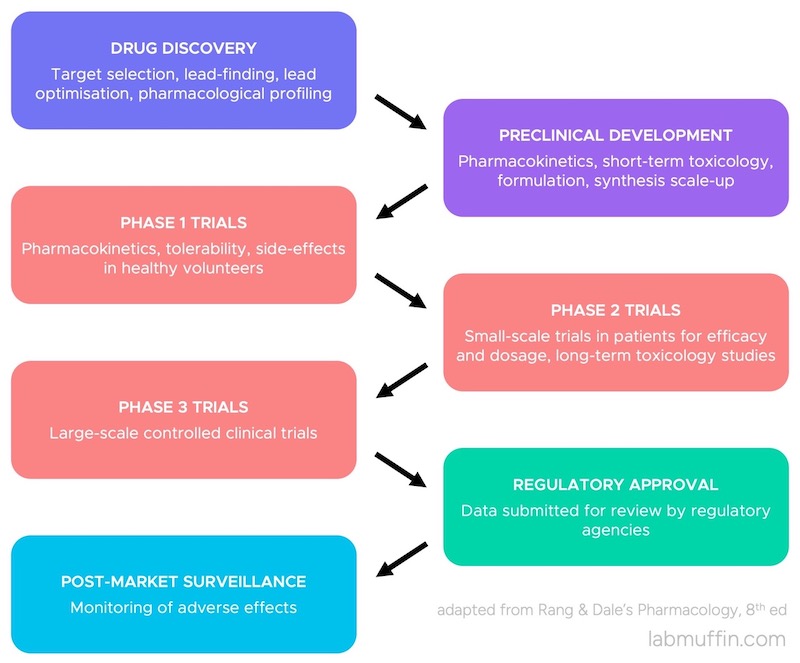
On the other hand, cosmetics are defined as products that have more superficial effects – they just change the appearance of skin.
Retinol is classified as a cosmetic ingredient, so this makes it seem like retinol doesn’t work. It feels a lot like this:


What this is missing: a lot of the time, whether something becomes a drug isn’t about what it does to your body.
This drug versus cosmetic definition was created almost 100 years ago before we, as a species, realised bloody everything can change the structure and function of skin, at least in little ways – including water, and even just a piece of plastic tape.
Related post: The Only Skincare That Works According to Science? ASAP Science video response
I mean, these effects of occlusion are pretty intense for something that literally just sits on top of your skin, and doesn’t even go in:

And some ingredients are drugs in one country and cosmetics in another. It’s not like you can tell molecules what they can and can’t legally do to your skin after you’ve crossed the border.
So this is a common misconception:
“It is illegal in the United States for cosmetics to have a structure or function effect,” the cosmetic chemist elaborates.
Mary Schook, an aesthetician and product formulator, clarifies that per guidelines from the country’s Food & Drug Administration, “a cosmetic brand can only enhance or beautify, not treat or cure.” This is why skincare brands often employ aesthetic-focused marketing language; for example, “makes skin look healthier” or “reduces the appearance of acne.”
from Jessica Defino, “The ‘Science of Skincare’ Isn’t Science or Care”
How cosmetics are legally defined doesn’t match what cosmetics are actually doing to skin. But at the same time, it would be ridiculous and unnecessary to regulate water and sticky tape as drugs.
The way it mostly works in practice: cosmetics aren’t meant to claim that they change the structure or function of skin. And if cosmetic companies start selling really potent, potentially dangerous stuff, then the government steps in.
Historical quirks
But with retinoids, you might be wondering: they all kind of do the same thing, they work in the same way… but some of these made it to the drug side. Why is retinol a cosmetic?
This is a really good question, and it’s almost entirely a historical accident. From an MD Edge podcast with a dermatologist who was involved in the original research on retinoids for skin:
Dr Leslie Baumann: How did it come about that retinol is over the counter [cosmetic] and retinoids are prescription?
Dr James Leyden: The reason is, retinol has been in a lot of products, including cosmetics, for a long time, for its antioxidant properties. So it was already in over the counter products.
And then as it became apparent, and the work of Sewon Kang and John Voorhees when they showed retinol was a prodrug of retinoic acid… once we became aware of all that, then obviously retinol might have some… some benefit and it clearly does, and there are many, many good, really good studies that show there’s definitely benefit.
So the FDA couldn’t say, well, we can’t allow this over the counter because it’s already there, you know, and for another reason, just at a different concentration…
So there are some good reasons why retinol isn’t legally classified as a drug. But if it works… where are the scientific studies?
Cash Rules Everything Around Me
Here’s the thing with the peer-reviewed scientific literature: not everything has an equal chance of ending up there. And there are many good reasons why we don’t see high quality, peer-reviewed clinical trials on cosmetics that often.
Yes, one of these reasons is that a lot of stuff probably doesn’t work that well.
But a really important reason that gets missed a lot is: there’s just not that much incentive to do them.
If you look at how research works, and how the cosmetic industry works, it makes a lot of sense.
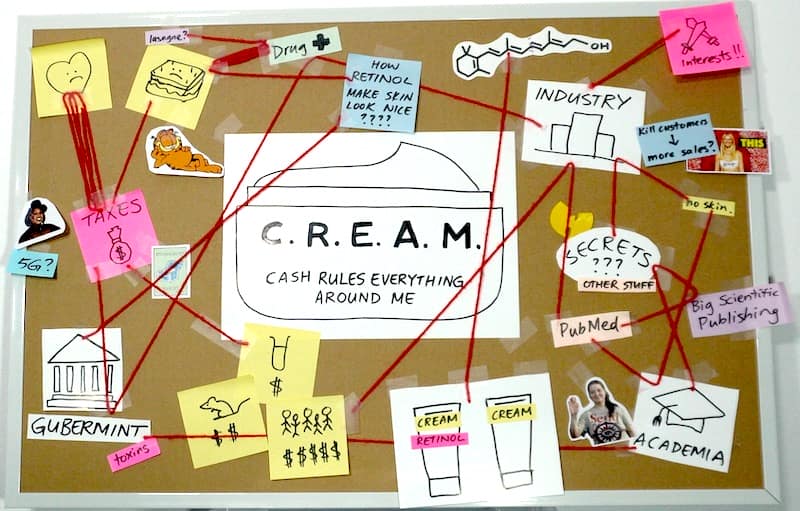
Funding sources
Research costs a lot, especially clinical trials. Most peer-reviewed research is funded by taxes. It’s a lot easier to get funding to research heart disease than skin. And within skin research, it’s a lot easier to get money to study drugs approved to treat medical conditions, than How Retinol Make Skin Look Nice.

That means more skin studies are funded by skincare and ingredient companies, which isn’t as nice because there are potential conflicts of interest. But even if the studies are all 10000% honest, this still changes the types of studies that get funded.
As someone who owns skin, I would love more high quality clinical trials on skincare. It’s nice, simple, direct evidence – you literally put the thing on a bunch of faces, and see if something happens.
But that’s not how companies think. They don’t have skin.

Back to the government…
Regulatory pressure
Companies are going to do less trials on cosmetics. Clinical trials are actually needed before you sell a drug – but cosmetics don’t need them. If a company doesn’t need to pay for an expensive thing, they’re less likely pay for the expensive thing.
And it is expensive. Back when I had less back pain (2004-2012), a proper clinical trial for a dermatology drug cost $11.5 million USD on average (not adjusted for inflation).
That drug vs cosmetic definition means that even if a cosmetic ingredient “did something” in a study, companies can’t just put it on a billboard. So it’s not exactly the most guaranteed return on investment, compared to everything else they could be spending their money on.

Publication bias
Let’s say a company has decided to pay for a clinical trial – and they often do, despite all these barriers. Well-designed experiments are a great way to test what works, and make better products. Yay scientific method!
But publishing a study in a peer-reviewed journal is a choice. The company could also… not publish it, and just use the findings to improve their products, instead of giving free information to their competitors.
And this happens a LOT. I’ve consulted for some companies that invest heavily in R&D, and it is extremely obvious that what we actually see is a tiny sliver of the actual research they’ve done.
Related post: Behind the Scenes at Procter & Gamble’s Singapore Innovation Centre
Now, for academic scientists, doing good research and not publishing it in peer-reviewed journals is super weird (I know because academia is where I was made) – and it’s because the incentives are so different there.
Part of why academics publish papers is because it contributes to scientific knowledge – it’s just “how science is done” (that idea drives a lot of industry publishing too).
But a very big part of it for academics is: that it’s how you keep your job. It’s “publish or perish” – your peer-reviewed publications are really important when universities decide who gets hired and who gets promoted, and when research funding gets decided.

I’d even argue that confidential internal company data is probably a lot more accurate and reliable than academic research, on the whole – despite the lack of peer review, and despite the fact it’s funded by the company – because of this incentive structure.
This huge pressure to “publish or perish” is a big reason for fake results, because negative findings are less likely to be published. Plus it’s pretty unlikely anyone will try to replicate your work, because replications are also less likely to be published.
On the other hand, if you’re doing research within a company and you get an amazing result, it’s probably going to get investigated more to try to turn it into a product. So if it’s a dud, it’ll be found out pretty quickly.
Diminishing returns
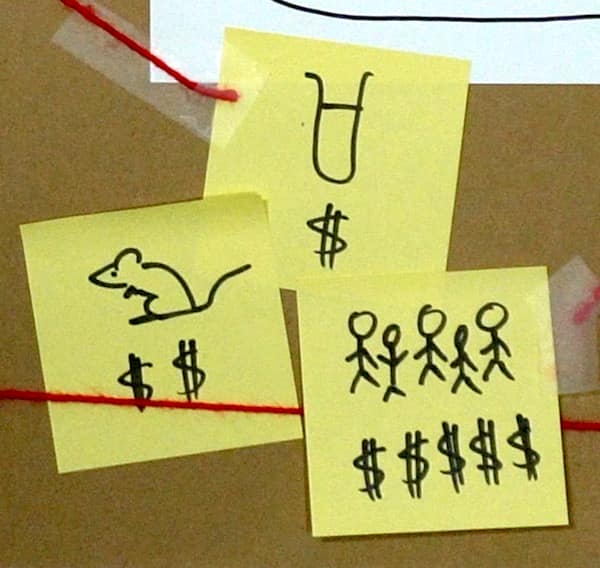
So the peer-reviewed studies that do come out are going to lean towards maximum benefit to the company, at minimal cost. And a lot of the time, this is the opposite of good study design.
For example:
A “well-designed” trial to test retinol would mean it’s tested on its own against a vehicle control (i.e. cream with retinol vs exact same cream without retinol), to make sure it’s the retinol working.

If you’re Big Cosmetic Company, you’ve just paid a ton of money to show that retinol, an ingredient that every other company also has in their products, works.
On the other hand, you could spend the same money and test your specific retinol formula against a plain cream… or even against nothing (just to rig it a bit, and make your product look better).
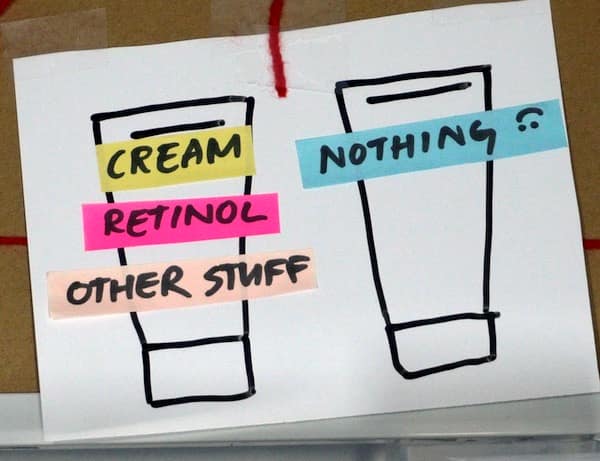
So if you’re looking at it purely as an investment, it kind of leans very one way.
Related post: Science vs Anecdotal Evidence and Reviews
“Well-designed” also usually means more participants. Repetition is really important in science – the more times you test something, the more confident you can be that a result isn’t a one-off fluke.
In drug trials they’ll test on hundreds or thousands of people. Most cosmetic trials have less than 30 people – why would you pay for 300 people when you could just use 30 and still be doing more than everyone else?
So there are a lot of reasons why a cosmetic company probably isn’t going to pay big bucks for a well-designed clinical trial on retinol that they’ll publish in a peer-reviewed journal, that ends up on PubMed.

The only real incentive would be to get new customers who are nerdy enough to be impressed by a well-designed clinical trial, but don’t already think that retinol works.
This is a very, very tiny group of people – again, just an awful investment. Especially since pretty much everyone who understands retinoid pharmacology accepts that at least some retinol products work…
The evidence for retinol
So, why? Why do so many scientists think retinol works despite the lack of high quality clinical trials?
Even Kligman who invented tretinoin, and has a reputation for hyping it up maybe a bit too much, says retinol (a competing ingredient) works:
…recent investigations with retinol in the appropriate concentration and vehicle have shown that it is as effective as tretinoin for the same indications. The limiting factor of the early work was the instability of retinol – it was quickly rendered inactive by oxidation. Thus the formulated products had no shelf life. Because retinol is metabolically converted to retinoic acid, one would predict efficacy if oxidative degradation could be prevented.
I love clinical trials – I think all scientists do. Thing go on face, face change – nice.
But I think sometimes we forget that the whole point of science is to build an understanding of the world, so we don’t have to directly test everything.
Clinical trials and scientific reasoning
Clinical trials have been important for so long because human biology is complex. They aren’t used for better understood areas of science, like astrophysics.
It can be hard to accurately predict what happens when you take a pill – the active ingredient has to travel through your digestive system, get absorbed, go into your blood, get processed by your liver, and end up in the right part of your body before it has a chance to work.
But skin products are less complex. They just have to go through a relatively short distance into the skin, so it’s a lot easier to work out what’s going on from other types of evidence.
Our scientific understanding of human biology is improving as well. In toxicology, for example, there’s been increasing use of New Approach Methods (NAMs) instead of directly testing on people or animals.
And this sort of scientific reasoning and extrapolation is implicitly used in clinical trials too!
If a drug works in a clinical trial in one place, we assume that it’s going to work in lots of other people, in lots of other places, maybe years after the original trials.
That’s because (thanks to science) we know that human biology doesn’t evolve that quickly, drug molecules aren’t really affected by where you are. Clinical trials for tretinoin didn’t tell people whether they should sit or stand while applying it, because again, thanks to science, we know that won’t change how it works.
So there’s actually a lot of other scientific evidence on retinol, and put together, it builds a really convincing case that if there was incentive to do high quality clinical trials, they would find that retinol works.
Other forms of evidence
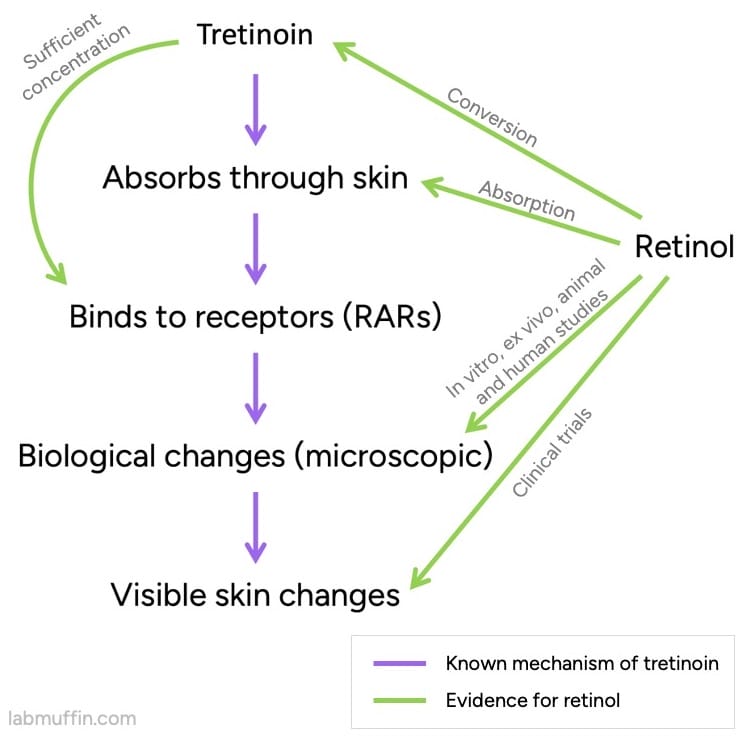
One of the key pieces of evidence is that in your skin, retinol can be converted to tretinoin, an approved drug. (As Dr Leyden said, it’s a prodrug of tretinoin.)
There’s a lot of evidence that tretinoin works, and we know a lot about how it works (its pharmacological mechanism of action).
Related post: 7 Common Retinoid Skincare Myths, Busted
So far, this isn’t a slam dunk – we still need the tretinoin that’s formed from retinol to end up in the right place (close to the retinoic acid receptors or RARs), and at a high enough concentration. Luckily, we have evidence for this!
We also have evidence that applying normal amounts of retinol to skin will change the levels of particular substances inside skin. These are the same ones that change with tretinoin, and are expected to be an important part of how tretinoin works.
A non-exhaustive list:
- Increased collagen or procollagen (Varani 2000, Kim 2010, Randhawa 2015, Kong 2016, Shao 2017)
- Increased CRABP-II mRNA or protein levels (Kang 1995, Duell 1996, Kafi 2007, Bellemère 2009)
- Decreased MMPs (Varani 2000, Kim 2010)
- Increased elastin or elastin components (fibrillin, tropoelastin) (Rossetti 2011, Shao 2017, Romana-Souza 2019, Mellody 2022)
- Increased epidermal thickness (Connor 1988, Kang 1995, Duell 1996, Shao 2017, Kong 2016, Romana-Souza 2019)
- Increased EGF or EGFR (Bellemère 2009, Romana-Souza 2019)
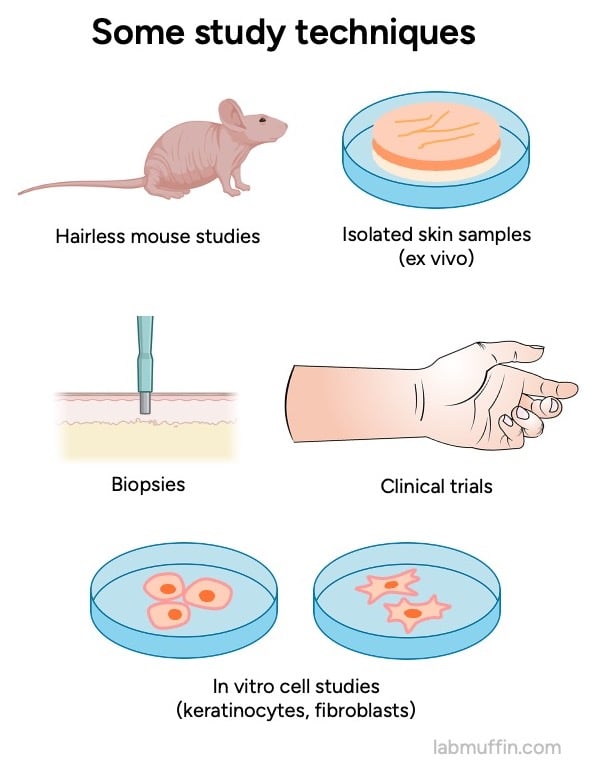
And for each of these we have multiple types of evidence: studies on mice, on people (biopsies taken during testing), ex vivo studies on bits of skin removed from people, in vitro studies on different types of isolated human skin cells (keratinocytes, fibroblasts).
You might have also experienced some evidence for yourself. If you’ve ever used a bit too much retinol, you might’ve noticed a special kind of peeling. It’s not the same as the peeling you get from other irritating products – there’s a delay, and it feels like you just have a bit too much skin.

This is a pretty unique side effect of retinoids, with a special name (retinisation or retinoid dermatitis), and it happens because of how retinoids work.
Related post: How to Start on Tretinoin (Retin-A) and Retinol
Activating retinoic acid receptors triggers the epidermal growth factor (EGF) pathway, which leads to more cells being produced in the top layers of your skin. (Special mutant mice that don’t have retinoic acid receptors don’t have this peeling side effect with tretinoin.)
There’s also the not-as-high-quality clinical trials we do have. Despite all this lack of incentive making the bar much higher for conducting and publishing them, retinol still has a lot that show promising results.
And all this evidence includes studies from independent research groups, without funding from cosmetic companies. A lot of the studies funded by cosmetic companies were done in conjunction with independent academic researchers. And industry funding for the studies comes from lots of different cosmetic companies.
| Study | Type | Industry? |
|---|---|---|
| Kawada 2009 | Clinical trial | No |
| Kikuchi 2009 | Clinical trial | One author J&J advisor, J&J funded |
| Draelos 2020 | Clinical trial with biopsies | One author from TOPIX |
| Bellemere 2009 | Clinical trial with biopsies | All authors from J&J |
| Li 2017 | Clinical trial and in vitro | All authors from J&J |
| Babcock 2015 | Clinical trial | Two authors from SkinMedica (Allergan) |
| Ho 2012 | Clinical trial | Lead author from SkinMedica |
| Bouloc 2015 | Clinical trial | One author from Vichy, Vichy funded |
| Kafi 2007 | Clinical trial with biopsies | No |
| Randhawa 2015 | Clinical trial with biopsies | Most authors from J&J |
| Tucker-Samaras 2009 | Clinical trial | Most authors from J&J |
| Kong 2015 | Clinical trial with biopsies | Most authors from Amway |
| Oblong 2005 | Clinical trial and biopsies | All authors from P&G |
| Pierard-Franchimont 1998 | Clinical trial | Most authors from J&J |
| Jang 2023 | Clinical trial | Most authors from Amorepacific |
| Mellody 2022 | In vivo human (biopsies) | Some authors from Boots, part Boots funded |
| Kim 2010 | In vivo mouse | No |
| Connor 1988 | In vivo mouse | No |
| Kang 1995 | In vivo human (biopsies) | No |
| Varani 2000 | In vivo human (biopsies) | Part J&J funded |
| Shao 2016 | In vivo human (biopsies) | Part J&J funded |
| Romana-Souza 2019 | Ex vivo and in vitro human | No |
| Rossetti 2011 | Ex vivo and in vitro human | Most authors from J&J |
| Kurlandsky 1994 | In vitro human | Part J&J funded |
| Bailly 1998 | Ex vivo and in vitro human | Most authors from pharma |
| Duell 1996 | In vivo human (biopsies) | No |
| Zouboulis 1999 | In vitro human | No |
| Randolph 1993 | In vitro human | No |
| Duell 1997 | In vivo human (biopsies) | Part J&J funded |
| Kim 2023 | In vitro human | Most authors from Amorepacific |
There are also studies on other cosmetic retinoids, with results that wouldn’t make sense unless retinol worked.
Related post: How Long Do Retinoids Take to Work?
It’s just really, really unlikely that all of these scientists and companies are part of some huge conspiracy that’s been going on for almost 30 years.
So in this situation where there are very good practical reasons why high quality peer-reviewed clinical trials don’t exist, and there’s an overwhelming amount of other scientific evidence that something works, I think it’s very reasonable to conclude that retinol probably works.
Now, I’m not saying that you can’t choose to only use ingredients with well-designed peer-reviewed clinical trials – that’s a completely reasonable personal choice.
But saying that the only ingredients that work are the ones with high quality peer-reviewed clinical trials, and anything without that specific type and level of evidence is completely useless, when there are good reasons why they’re not done… THAT is actually unscientific.
And there are some upsides that cosmetic retinoids have over drug retinoids, which I’ll be talking about in a different post.
Related post: Bakuchiol: Better Than Retinol?
What about other skincare ingredients?
I’ve mostly talked about retinol, the skincare ingredient with the most evidence behind it. But there are a lot of things we can take from this scenic journey that can help us find other effective ingredients.
This is the Lab Muffin Matrix, which we saw before in the context of L-ascorbic acid (vitamin C) products. It shows how to think about the evidence when deciding if a skincare product has a good chance of working.
Related post: Ultimate Vitamin C Skincare Guide Part 1: Ascorbic Acid

The left hand side is about ingredients:
Ingredient(s) theoretically work? You want to see if the active ingredient can theoretically work. This includes looking at things like chemical properties, mechanism of action, cell studies and animal studies.
Do we know how it works? What is it interacting with inside the skin? Can it get into skin?
Ingredient(s) actually work? You also want to see if the active ingredient has been tested to actually work on people. You want to look for studies where the ingredient’s been applied to actual people’s skin, like clinical trials and biopsy evidence.
The right hand side is about specific product formulas. Again, you want to see if the formula can theoretically work, and if that formula has actually been tested on people.
Some ingredients are easier to formulate with, so you don’t have to do as much guesswork here. But others are more annoying, like if they’re unstable, which a lot of retinoids are – I’ll be talking more about this in later posts, but a good general guideline is to look for products from companies who invest in product development.
And for all of these, if there are more different types of evidence, more different scientists contributing to this body of evidence, more repetition of findings, then you can be more confident about the conclusion.
References
Skin Care: Is Anti-Aging a Scam? Science Vs Podcast. May 18, 2023. Accessed October 18, 2023.
Spierings NMK. Evidence for the Efficacy of Over-the-counter Vitamin A Cosmetic Products in the Improvement of Facial Skin Aging: A Systematic Review. J Clin Aesthet Dermatol. 2021;14(9):33-40.
Bertin C, Zunino H, Lanctin M, et al. Combined retinol-lactose-glycolic acid effects on photoaged skin: a double-blind placebo-controlled study. Int J Cosmet Sci. 2008;30(3):175-182. doi:10.1111/j.1468-2494.2008.00441.x
Looking back on retinoid discovery and development with Dr. James Leyden. Dermatology Weekly Podcast (MDedge). October 15, 2020. Accessed October 18, 2023.
Leow YH, Maibach HI. Effect of occlusion on skin. J Dermatolog Treat. 1997;8(2):139-142. doi:10.3109/09546639709160288
Sertkaya A, Wong HH, Jessup A, Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials. 2016;13(2):117-126. doi:10.1177/1740774515625964
Baumann LS. Retinoids. In: Baumann LS, Rieder EA, Sun MD, eds. Baumann’s Cosmetic Dermatology. 3rd ed. McGraw Hill; 2022:615-630.
Kligman AM. The growing importance of topical retinoids in clinical dermatology: a retrospective and prospective analysis. J Am Acad Dermatol. 1998;39(2 Pt 3):S2-S7. doi:10.1016/s0190-9622(98)70437-2
Renaud JP, Rochel N, Ruff M, et al. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature. 1995;378(6558):681-689. doi:10.1038/378681a0
Retinol studies
Babcock M, Mehta RC, Makino ET. A randomized, double-blind, split-face study comparing the efficacy and tolerability of three retinol-based products vs. three tretinoin-based products in subjects with moderate to severe facial photodamage. J Drugs Dermatol. 2015;14(1):24-30.
Bailly J, Crettaz M, Schifflers MH, Marty JP. In vitro metabolism by human skin and fibroblasts of retinol, retinal and retinoic acid. Exp Dermatol. 1998;7(1):27-34. doi:10.1111/j.1600-0625.1998.tb00299.x
Bellemère G, Stamatas GN, Bruère V, Bertin C, Issachar N, Oddos T. Antiaging action of retinol: from molecular to clinical. Skin Pharmacol Physiol. 2009;22(4):200-209. doi:10.1159/000231525
Bouloc A, Vergnanini AL, Issa MC. A double-blind randomized study comparing the association of Retinol and LR2412 with tretinoin 0.025% in photoaged skin. J Cosmet Dermatol. 2015;14(1):40-46. doi:10.1111/jocd.12131
Connor MJ. Oxidation of retinol to retinoic acid as a requirement for biological activity in mouse epidermis. Cancer Res. 1988;48(24 Pt 1):7038-7040.
Draelos ZD, Peterson RS. A Double-Blind, Comparative Clinical Study of Newly Formulated Retinol Serums vs Tretinoin Cream in Escalating Doses: A Method for Rapid Retinization With Minimized Irritation. J Drugs Dermatol. 2020;19(6):625-631. doi:10.36849/JDD.2020.5085
Duell EA, Derguini F, Kang S, Elder JT, Voorhees JJ. Extraction of human epidermis treated with retinol yields retro-retinoids in addition to free retinol and retinyl esters. J Invest Dermatol. 1996;107(2):178-182. doi:10.1111/1523-1747.ep12329576
Duell EA, Kang S, Voorhees JJ. Unoccluded retinol penetrates human skin in vivo more effectively than unoccluded retinyl palmitate or retinoic acid. J Invest Dermatol. 1997;109(3):301-305. doi:10.1111/1523-1747.ep12335788
Feng X, Peng ZH, Di W, et al. Suprabasal expression of a dominant-negative RXR alpha mutant in transgenic mouse epidermis impairs regulation of gene transcription and basal keratinocyte proliferation by RAR-selective retinoids. Genes Dev. 1997;11(1):59-71. doi:10.1101/gad.11.1.59
Ho ET, Trookman NS, Sperber BR, et al. A randomized, double-blind, controlled comparative trial of the anti-aging properties of non-prescription tri-retinol 1.1% vs. prescription tretinoin 0.025%. J Drugs Dermatol. 2012;11(1):64-69.
Jang SI, Jung YC, Suk J, et al. A long term study of the difference in efficacy and effect rate of various concentrations of retinol (1500-6600 IU) in middle aged women. Arch Dermatol Res. 2023;315(5):1323-1332. doi:10.1007/s00403-022-02520-2
Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol. 2007;143(5):606-612. doi:10.1001/archderm.143.5.606
Kang S, Duell EA, Fisher GJ, et al. Application of retinol to human skin in vivo induces epidermal hyperplasia and cellular retinoid binding proteins characteristic of retinoic acid but without measurable retinoic acid levels or irritation. J Invest Dermatol. 1995;105(4):549-556. doi:10.1111/1523-1747.ep12323445
Kawada A, Konishi N, Momma T, Oiso N, Kawara S. Evaluation of anti-wrinkle effects of a novel cosmetic containing retinol using the guideline of the Japan Cosmetic Industry Association. J Dermatol. 2009;36(11):583-586. doi:10.1111/j.1346-8138.2009.00716.x
Kikuchi K, Suetake T, Kumasaka N, Tagami H. Improvement of photoaged facial skin in middle-aged Japanese females by topical retinol (vitamin A alcohol): a vehicle-controlled, double-blind study. J Dermatolog Treat. 2009;20(5):276-281. doi:10.1080/09546630902973987
Kim BH. Safety Evaluation and Anti-wrinkle Effects of Retinoids on Skin. Toxicol Res. 2010;26(1):61-66. doi:10.5487/TR.2010.26.1.061
Kim JE, Kim WH, Kim S, et al. Bioconversion of retinol and its cell barrier function in human immortalized keratinocytes cells and artificial epidermis-dermis skin. Exp Dermatol. 2023;32(6):822-830. doi:10.1111/exd.14781
Kong R, Cui Y, Fisher GJ, et al. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J Cosmet Dermatol. 2016;15(1):49-57. doi:10.1111/jocd.12193
Kurlandsky SB, Xiao JH, Duell EA, Voorhees JJ, Fisher GJ. Biological activity of all-trans retinol requires metabolic conversion to all-trans retinoic acid and is mediated through activation of nuclear retinoid receptors in human keratinocytes. J Biol Chem. 1994;269(52):32821-32827. DOI: 10.1016/S0021-9258(20)30065-X
Li WH, Wong HK, Serrano J, et al. Topical stabilized retinol treatment induces the expression of HAS genes and HA production in human skin in vitro and in vivo. Arch Dermatol Res. 2017;309(4):275-283. doi:10.1007/s00403-017-1723-6
Mellody KT, Bradley EJ, Mambwe B, et al. Multifaceted amelioration of cutaneous photoageing by (0.3%) retinol. Int J Cosmet Sci. 2022;44(6):625-635. doi:10.1111/ics.12799
Oblong J E, Saud A, Bissett D L, Zhu C. Topical Retinyl Propionate Achieves Skin Benefits with Favorable Irritation Profile. In: Elsner P, Maibach HI, eds. Cosmeceuticals and Active Cosmetics: Drugs Versus Cosmetics. 2nd ed. CRC Press; 2005:441-464. https://doi.org/10.1201/NOE0824759438
Piérard-Franchimont C, Castelli D, Cromphaut IV, et al. Tensile properties and contours of aging facial skin. A controlled double-blind comparative study of the effects of retinol, melibiose-lactose and their association. Skin Res Technol. 1998;4(4):237-243. doi:10.1111/j.1600-0846.1998.tb00116.x
Randhawa M, Rossetti D, Leyden JJ, et al. One-year topical stabilized retinol treatment improves photodamaged skin in a double-blind, vehicle-controlled trial. J Drugs Dermatol. 2015;14(3):271-280.
Randolph RK, Simon M. Characterization of retinol metabolism in cultured human epidermal keratinocytes. J Biol Chem. 1993;268(13):9198-9205.
Rittié L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126(4):732-739. doi:10.1038/sj.jid.5700202
Romana-Souza B, Silva-Xavier W, Monte-Alto-Costa A. Topical retinol attenuates stress-induced ageing signs in human skin ex vivo, through EGFR activation via EGF, but not ERK and AP-1 activation. Exp Dermatol. 2019;28(8):906-913. doi:10.1111/exd.13675
Rossetti D, Kielmanowicz MG, Vigodman S, et al. A novel anti-ageing mechanism for retinol: induction of dermal elastin synthesis and elastin fibre formation. Int J Cosmet Sci. 2011;33(1):62-69. doi:10.1111/j.1468-2494.2010.00588.x
Shao Y, He T, Fisher GJ, Voorhees JJ, Quan T. Molecular basis of retinol anti-ageing properties in naturally aged human skin in vivo. Int J Cosmet Sci. 2017;39(1):56-65. doi:10.1111/ics.12348
Tucker-Samaras S, Zedayko T, Cole C, Miller D, Wallo W, Leyden JJ. A stabilized 0.1% retinol facial moisturizer improves the appearance of photodamaged skin in an eight-week, double-blind, vehicle-controlled study. J Drugs Dermatol. 2009;8(10):932-936.
Varani J, Warner RL, Gharaee-Kermani M, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114(3):480-486. doi:10.1046/j.1523-1747.2000.00902.x
Varani J, Zeigler M, Dame MK, et al. Heparin-binding epidermal-growth-factor-like growth factor activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side-effect of retinoid therapy. J Invest Dermatol. 2001;117(6):1335-1341. doi:10.1046/j.0022-202x.2001.01564.x
Zouboulis CC, Seltmann H, Sass JO, et al. Retinoid signaling by all-trans retinoic acid and all-trans retinoyl-beta-D-glucuronide is attenuated by simultaneous exposure of human keratinocytes to retinol. J Invest Dermatol. 1999;112(2):157-164. doi:10.1046/j.1523-1747.1999.00496.x






Great work as always Michelle 🙂 It’s a Catch 22 – if it works it’s medicine, but if it’s medicine then your market changes. So the vested interests here are huge! Personally I’ve had some great results with retinol so I’m convinced of its effectiveness – nothing else has peeled off a layer of skin quite like it.
Hi Dr Michelle, I am a subscriber to your YT channel. I am 67 and when I was a youngster I had bad acne, not cystic but bad enough that my doctor prescribed Retin A cream. I was advised of all the risks eg sun exposure and falling pregnant. Well the product did the trick and my acne after a while (yikes don’t remember how long it took) but my face cleared up. I continued to look after my skin eg sunscreen etc, wearing a hat you know the drill. Well my point is my skin is very unlined now. Even my doctor comments on it and tells me she can’t believe I am 67! Oh by the way I am a firm believer in using Retinol products, I am proof!
Love your work,
Cheers,
Trish
What a brilliant article! It’s great to read a common sense, balanced, science-informed view of the value of skincare.
I searched your site for info on estrogen creams (like the one Alloy offers) for perimenopausal and menopausal women but doesn’t appear the topic has been addressed. Would be curious to hear your thoughts on these creams. Are they just a gimmick?