Sulfates, fatty alcohols, silicones, preservatives… what are they doing in your shampoo and conditioner, and do you need to avoid them?
Today’s post is a deep dive into some of the key ingredients in haircare products. The video version is here if you prefer that format!
Note: This post is based on a video sponsored by The Ordinary, but this post is not sponsored.
Science of Shampoo
Let’s start with shampoo.
To me, the best way to approach analysing formulas is to consider: What is the point of this product? What is its function?
Shampoo obviously cleans your hair and scalp. Just like skin, your scalp produces sebum, which we usually think of as oil. It keeps your skin and scalp nice and soft, and also travels along the hair to keep that soft too.
But sometimes we have too much oil (some of us (me) more than others), and it also tends to stick to other things like dirt, dust, skin flakes and pollution. You might also use styling products that need to be washed off.
The problem is: if you just use water to try to degrossify the situation a bit, it usually doesn’t work very well. Oil and water don’t mix.

So to make them mix, we use a shampoo, and the key cleaning ingredients in shampoos are surfactants.
What are surfactants?
Surfactants are really useful ingredients because they have a head that likes water, and a tail that likes oil.
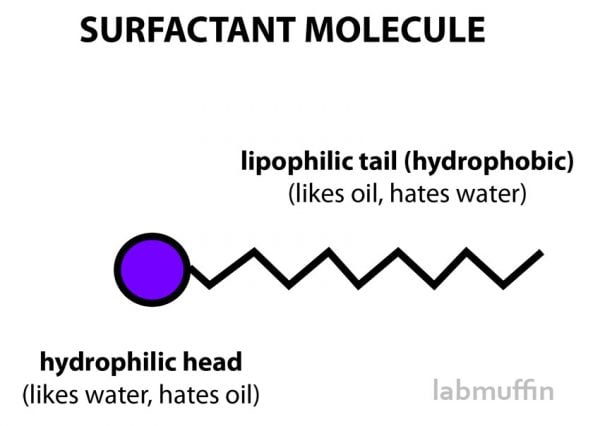
So instead of repelling each other, surfactants allow the oil to basically be smuggled into the water in lots of tiny droplets, and get washed away down the drain.
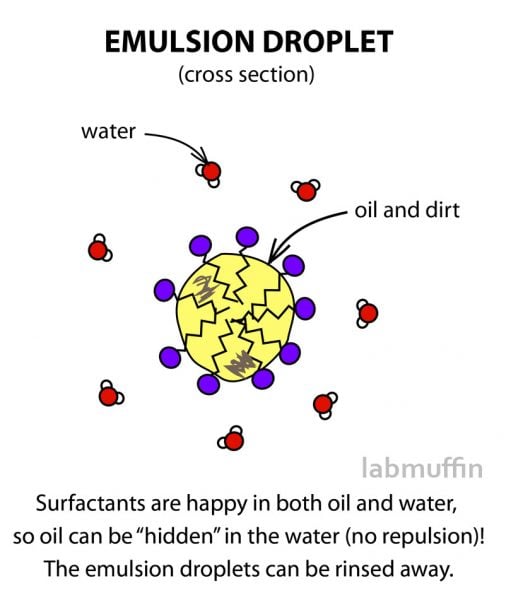
There are surfactants in tons of different products, because getting oil and water to stay together is really useful!
Related post: Surfactants Are Everywhere, aka Stop Being Terrified of Chemicals
For cleaning, the best surfactants are anionic surfactants. These have a negatively charged head, and they’re used in tons of cleaning products. They’re in shampoo (obviously), but also laundry detergent, dishwashing detergent, body washes, and face cleansers.
Related post: Are You Washing Your Face Wrong? Busting Cleanser Myths (with video)
Some anionic surfactants you might’ve seen in your shampoos and cleansers include sulfates, sarcosinates, taurates and isethionates. Traditional soaps also have anionic surfactants (carboxylates).
Related post: Make Your Own Soap! Part 1: The Chemistry Behind Soap Making
Spotlight on sulfates
So that’s what sulfates are doing – but why are people talking about “sulfate-free”?
Well, like a lot of other ingredient-avoidance trends, a lot of it is based on misinformation. Once you start digging into the science behind shampoos, you start to realise that limiting yourself to “sulfate-free” shampoos doesn’t really make sense.
Sulfate surfactants are a diverse group of ingredients that behave in different ways. And on top of that they can be combined in formulas in lots of different ways to give very different shampoos.
It’s a bit like eggs in a recipe. Just seeing eggs in the ingredients list doesn’t tell you whether they used the whites or the yolks or a mix, how the food tastes or whether you’ll like it (unless you have an egg allergy, in which case you should probably avoid it unless you like pain, or you’re vegan).

So generalising sulfates in products doesn’t really work. It isn’t like shampoos with sulfates are always harsher, more irritating, or strip colour faster than sulfate-free shampoos. There’s a lot of overlap!
Sulfates are some of the most commonly used anionic surfactants in personal care products. The two you’ll see most in shampoos are sodium lauryl sulfate (SLS) and sodium laureth sulfate (SLES). These sound pretty similar, but they’re actually very different.
Sodium lauryl sulfate (SLS)
Sodium lauryl sulfate (SLS) is the more famous sulfate, and it has a bad reputation, which has now spread to other sulfates. It has a narrow structure that means it can potentially absorb into skin more easily and keep irritating for longer.
If it’s in a formula on its own at a high concentration it’ll probably be quite irritating, and this is one of the big reasons why it has this “irritant” reputation. In peer-reviewed studies and in dermatology patch tests, SLS is usually used by itself to make sure it’s the specific ingredient someone is reacting to. But this also means it’s not in its usual environment: in a product with a lot of other ingredients that change how it interacts with skin and hair.
Some of the formulating tricks that are usually used in SLS products:
Creating mixed micelles by combining it with other surfactants – this makes SLS less likely to wander off on its own into your skin or hair. It’s peer pressure, but for molecules.
Adding polymers that act as a “raft” to hold onto free SLS molecules – again, so it can’t run away into your skin or hair.
Adding skin protecting ingredients like humectants and antioxidants which can prevent or reverse some of the irritation.
Related post: Are You Washing Your Face Wrong? Busting Cleanser Myths (with video)
Sodium laureth sulfate (SLES)
Fun fact: Sodium laureth sulfate (SLES) actually isn’t one single ingredient! It’s a category of ingredients that are all listed under the same name in an ingredients list. (Ingredient lists don’t really lie, but they don’t really tell you the full story either!)
SLES is ethoxylated, which means it’s SLS with a bunch of ethoxy units inserted next to the sulfur.
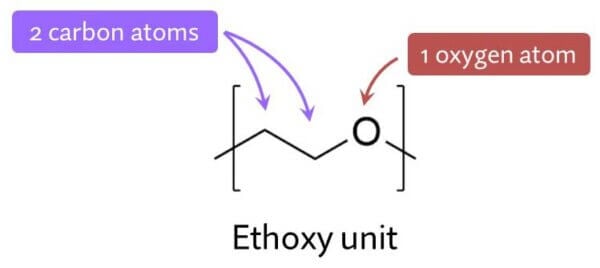
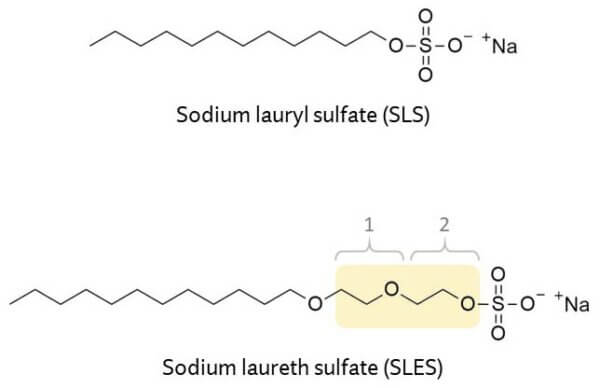
The number of units can change, and this changes how the specific type of SLES interacts with everything else: water, oil and other surfactant molecules. Having these ethoxy units means less SLES will wander off on its own (and into your skin) compared to SLS.
Ethoxylation also makes the molecule bigger, which means it doesn’t penetrate skin as easily and is more gentle. It’s a bit like walking through a crowd – if you want to get through the crowd faster, you try to make yourself smaller, and you don’t spread your arms out. Your skin is actually made up of tons of molecules crowded together, so larger molecules don’t penetrate as deeply.
One study found that SLES was gentler than SLS, as well as a whole bunch of non-sulfate surfactants that you often find in sulfate-free products (disodium cocoamphodiacetate, sodium lauroamphoacetate and sodium methyl cocoyl taurate).
And on top of this, formulators can use the same formulating tricks in SLES products mentioned earlier to make them even gentler.
Colour fading
I have a strong personal interest in preventing hair colour fading, as you can probably tell.

When I first dyed my hair 4 years ago, I used sulfate-free shampoos, because that’s what everyone says is best for keeping your hair colour bright…
Except I hated all the sulfate-free shampoos I tried! And I spent a lot of money on them too, because I went with a lot of very fancy brands out of sheer optimism.
I ended up just going back to sulfate shampoos, and I didn’t notice much of a difference with my hair colour fading.
Recently I saw some experimental data on colour fading from some hair researchers that confirmed what I noticed happening with my hair.
With colour fading, there are some differences between different shampoo formulas, and as you’d expect from all of what I’ve said so far, there’s a lot of overlap between sulfate-ful and sulfate-free shampoos. The pH of the shampoo actually made a bigger difference than whether or not it had sulfates – higher pH, more alkaline shampoos will leach some dyes out faster.
But by far the biggest thing that made a difference was just… water. Simply washing coloured hair in water, without shampoo, leaches out a ton of dye. The differences between different shampoos are tiny compared to washing versus not washing.
So my problem was that a lot of sulfate-free shampoos were formulated to be too gentle to clean my scalp properly. That meant I either washed my hair every day, or just suffered with sticky limp hair and an itchy scalp (and sometimes that happened anyway, even if I washed daily). Using a shampoo with sulfates allowed me to wash my hair less often, and so I ended up with less fading.
Sulfates are cheap… and that’s not bad
Sulfates are also quite cheap, which a lot of people paint as a bad thing – that companies are just using cheap ingredients to save money, even though there are better options. And this does happen, but it’s never the only reason formulators use a particular ingredient.
1. You have to make a product that works. The cheapest ingredient is just water, but if a company tried to sell plain water as a product, it would flop really hard.
2. Price is not directly correlated with quality. I’m sure we all have a favourite cheap meal we’d happily pick over a bunch of more expensive options. And shoutout to the video’s sponsor, The Ordinary – most of us have tried some super budget-friendly skincare products from The Ordinary that worked better than more expensive ones.
And sulfates do actually have really good properties that make them great in cleansing products. On top of just the price of the raw material, one of the reasons sulfates work out to be cheap is because they’re multifunctional.
They clean well, but they also foam well. More foam doesn’t actually mean a better clean, but psychologically if there isn’t much foam, most people don’t think it’s doing a good job.
Sulfates also help create a thicker texture, and you can usually add a little bit of salt to make a sulfate product thicken up more. Shampoo needs to be a bit thick so it sits nicely in your hand, and you can get it onto your hair effectively. It also makes it feel “more concentrated”.
A lot of other surfactants don’t have these properties, so if you take out the sulfates, you often have to add a bunch of other ingredients to boost foam and make it thicker, which adds to the cost.
Ingredient Breakdown: The Ordinary Sulphate 4% Cleanser
Let’s have a look at what’s in The Ordinary’s Sulphate 4% Cleanser for Body and Hair, which is a pretty stripped-back shampoo formula:

Ingredients: Aqua (Water), Sodium Laureth Sulfate, Polysorbate 20, Xanthan Gum, Tocopherol, Phytic Acid, Trisodium Ethylenediamine Disuccinate, Benzyl Alcohol, Ethylhexylglycerin, Phenoxyethanol, Chlorphenesin.
SLES: This is the anionic surfactant. It’s at 4% as you can tell from the name. This is pretty low for a shampoo, but this also doubles up as a body wash which usually has lower amounts, so keeps it gentle. They’re specifically using SLES-2, which means there’s 2 ethylene oxide units added to each molecule. This is the most common type of SLES used in shampoos, because it gives a good balance between cleaning, gentleness and cost.
Aside from the anionic surfactant, we can see some other ingredients:
Water: This is the first ingredient, and it’s pretty important. It keeps the surfactants spread out the right way when you put them on your hair or skin. If it’s too concentrated it’s also harsh – you don’t want 100% surfactant on your skin, trust me. Getting the right dilution also changes the thickness of the shampoo.
Polysorbate-20: This is another surfactant. It’s not as good at cleansing, and it’s usually used as a solubiliser, so it might be in there to help the tocopherol dissolve.
Xanthan gum: This thickens the texture.
Tocopherol: This is vitamin E, an antioxidant.
Preservatives (Benzyl Alcohol, Ethylhexylglycerin, Phenoxyethanol, Chlorphenesin): Stop bacteria and mould from having a fun multiplying festival in the shampoo
Chelating agents (Phytic Acid, Trisodium Ethylenediamine Disuccinate): These grab onto metal ions, which can make cleansers work worse, especially in hard water. They also help preservatives work better.
Like I said, this is a relatively simple formula. In other shampoos you’ll often find:
Other surfactants: Combining multiple surfactants makes a cleanser more gentle, some can also make it foam better or moisturise skin
Detangling ingredients: When hair is wet the cuticle sticks up more, so the surface is rougher and snags on other hairs more easily. Most shampoos have some ingredients that very thinly coat hair with a smoothing layer – the most common ingredients are polyquaterniums.
Colourants, fragrances, pearlising agents: To make the shampoo look and smell nice
pH adjuster: If the pH of the formula isn’t quite where you want it to be
The Ordinary Sulphate 4% Cleanser for Body and Hair review
My scalp gets dry and irritated from a lot of shampoos, so I was curious about how The Ordinary’s very simple formula would go for me.
This turned out to be really good for my scalp – one of the better tolerated shampoos I’ve used. Having multiple surfactants is usually good because it reduces irritation by making mixed micelles, which I talked about before, but they’ve gotten around that by using a low percentage of a gentle type of SLES.
There also isn’t any fragrance. I personally like a bit of fragrance in my hair products, but I know a lot of people want or need to avoid it. It’s actually pretty hard to find fragrance-free hair products, so I’m really happy to see this available as an option.
Science of Hair Conditioners
Now onto hair conditioners!
Hair conditioners have a few more functions than shampoo, and different people use them for different purposes – if you have short hair, you might not even need hair conditioner.
Conditioners can add shine, smooth over damage, and reduce tangling and static. To do this they leave a very thin coating on the hair strands.
There’s this myth that cheap conditioners coat hair but expensive ones don’t – no, all conditioners need to add a coating to work. Your hair is dead, and as it grows, it accumulates damage, and ends up chipped like this:

Hair conditioners smooth over this roughness by plugging in the gaps, so hair doesn’t snag and cause more of these bits to break off.
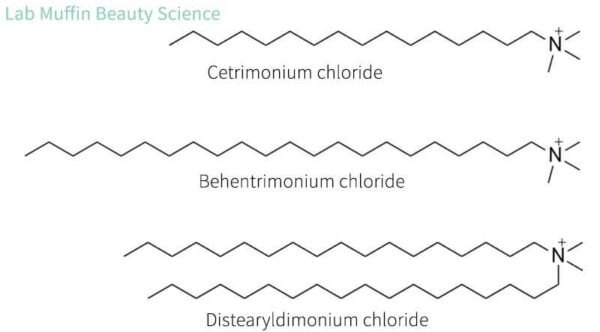
The main ingredients used in hair conditioners are cationic surfactants. These are a lot like the anionic surfactants we saw in shampoos, except they have a positively charged head.
When hair is wet, it gets a negative charge, and damaged bits of hair usually have a stronger negative charge. Opposite charges attract, so the positive heads of the cationic surfactants help them stick to the negatively charged hair.

The positive charges are also handy because once you have enough attached, the hair gets a slightly positive charge, which will actually repel extra cationic surfactants. This means that you end up with a thinner, more even film that feels silky, rather than the sort of film you get with a lot of oils (e.g. sebum) that’s heavy and weighs hair down.
The most common type of cationic surfactant you’ll see in conditioners are quaternary ammonium ions, nicknamed “quats”. You’ll see “onium” in a lot of their names. The Ordinary’s conditioner has 2% behentrimonium chloride.
Cationic surfactants are also why “co-washing” works, where you wash your hair with a conditioner. Cationic surfactants can still clean oil off your hair, even though they’re as effective.
In conditioners, cationic surfactants are usually combined with fatty alcohols. The most common is cetearyl alcohol, which is a mix of cetyl and stearyl alcohol. This makes the conditioner thicker so it’s easier to apply on your hair, but it also combines with the cationic surfactants to make an ordered arrangement called a lamellar structure. This is like a pile of flat tiles that can slide over each other and coat the hair.
These ingredients are also why a lot of people get breakouts on their back with conditioner – cationic surfactants are quite irritating, and fatty alcohols are pretty good at clogging pores. So make sure you scrub your back properly afterwards!
Ingredient breakdown: The Ordinary Behentrimonium Chloride 2% Conditioner
The Ordinary Behentrimonium Chloride 2% Conditioner is again pretty simple, with only 7 ingredients.

Ingredients: Aqua (Water), Cetearyl Alcohol, Behentrimonium Chloride, Cyamopsis Tetragonoloba (Guar) Gum, Phytic Acid, Phenoxyethanol, Chlorphenesin.
Apart from the cationic surfactant (Behentrimonium Chloride) and fatty alcohols (Cetearyl Alcohol), which are in pretty much every hair conditioner to form that thin conditioning film, there’s also:
Water: Like in shampoos, it’s there to make sure everything is at the right concentration and thickness for putting on your wet hair
Guar gum: This thickens the formula up
Preservatives (Phytic Acid, Phenoxyethanol, Chlorphenesin): To control bacteria and mould
With more complicated conditioners, you might get extra ingredients to coat the hair strands in different ways. Some of the common ones:
- Silicones: There’s a huge range of these, some tend to be heavier and people get the impression that they always weigh hair down, but this really isn’t a blanket rule. Some like my favourite amodimethicone are charged, so like the cationic surfactant you only get a thin film and any excess actually gets repelled.
- Polymers: e.g. polyquaterniums, similar to what’s used in shampoos to detangle and add volume
- Natural oils: e.g. coconut and argan oil. These tend to form thicker films on hair that can weigh it down, so they’re usually at low concentrations in conditioners.
- Hydrolysed proteins and amino acids: These can penetrate into the hair a little and add strength by plugging holes in the hair, as well as coating hair
Colourants, fragrances, pearlising agents: To make the conditioner look and smell nice
pH adjuster: If the pH of the formula isn’t quite where you want it to be
The Ordinary Behentrimonium Chloride 2% Conditioner review
I was actually pretty surprised by how well this worked on my hair. If you’ve followed my hair bleaching journey you’ll know I pretty much need specialised silicones like amodimethicone to make my hair not be straw.
Related posts: Amodimethicone: The Science of My Favourite Hair Ingredient
This conditioner detangled my hair really nicely when I was washing it, and it felt nice for about a day. But by day 2 my hair started feeling dry. I think this would’ve been fantastic for my hair pre-bleach, but now I need those extra damage-coating ingredients.
So I’d recommend trying this if your hair is not this level of damaged, if you want a conditioner that doesn’t weigh your hair down, or if you want something fragrance-free. Again, there are really not that many fragrance-free hair conditioners, so I’m really happy to see this as an option for people who want it.
I hope you enjoyed this deep dive into shampoos and conditioners – let me know if you’re interested in other product breakdowns!
The video version of this is sponsored, although this post isn’t sponsored. This post also contains affiliate links – if you decide to click through and support Lab Muffin financially (at no extra cost to you), thank you! For more information, see Disclosure Policy.
References







I haven’t been interested in the shampoos and conditioners from The Ordinary as I am pretty much stuck with a mixture of Olaplex and a random drugstore one that smells good and they don’t really spark my interest.
But I used to use sulfate-free shampoos too (I might even have some old blogposts up that need to be revised so they don’t share the misinformation…) and found it to be awful for my oily rots.
Thanks for the haircare products breakdown. It would be great if you would look into Hairprint True Hair Color Restorer. From their site: “Hairprint is a patented scientific breakthrough that restores gray hair to its true color. It is not a dye. It replenishes the natural pigment (eumelanin) found in brown and black hair that has turned gray. Hairprint is also a true protein treatment that will add body, strength, texture, and sheen while reducing frizz, and discoloration. Our simple, hypoallergenic, and odor-free formula produces color so natural, it defies detection. The treatment can be applied at home in about 75-90 minutes.”
okay learning about fatty alcohols made me realise why most moisturisers besides cicaplast and sukin gives me acne
Glad you are on the side of sulfates!
One little correction: the role of Polysorbate 20. “Polysorbate-20: This is another surfactant. It’s not as good at cleansing, and it’s usually used as a solubiliser, so it might be in there to help the tocopherol dissolve.”
It is not. A bit of help: it has something to do with SLS/SLES when combined with P20. What happens then?! 😊
I originally assumed the Polysorbate 20 was there to help form mixed micelles with the SLES and increase mildness, but I was told by a Deciem representative that wasn’t the case 🤷
Could you do a post on lamellar water? How is it different from/similar to amodimethicone? From what I’ve seen from your post on amodimethicone and other explanations of lamellar water, they both use the mechanism of positive/negative charge to coat the damaged portions of hair and avoid too much build-up of product.
Hello,
I want to clarify something said in this post:
You mentioned that chelating agents, such as phytic acid, make cleansers work worse, which is not my experience as a formulator. I usually find that they boost foam since they are binding to metals. Can you clarify if this is a typo, or why you deem them to be a hindrance to cleansing ability?
I meant that metal ions make cleansers work worse.
This whole series has been really helpful, thanks! If you’re looking for article ideas, I would love to hear about how lamellar water treatments work.