Surfactants Are Everywhere, aka Stop Being Terrified of Chemicals
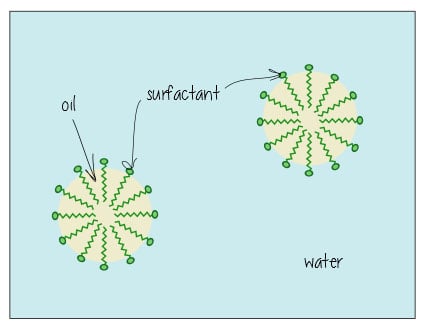
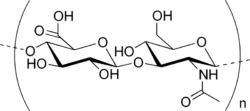
This post was originally published on the now sadly defunct awesome website The Toast in 2014. A reader suggested that I repost it on Lab Muffin… which I thought I did, but apparently that was all a fever dream. Here it is! There’s soap in your mayonnaise! As a scientist with a degree in chemistry, the surge in chemophobia over …