I’ve been a little frustrated with what scientific concepts I can get through in words and crudely drawn illustrations on this here blog. When I teach at my day job, I do a lot of hand-waving, emphatic underlining, symbolic gesturing and smashing together of whiteboard markers, and not having the ability to show movement sometimes feels a bit like I have one arm tied behind my back. So I’ve decided to venture into video, which has allowed me to whip up some very crude animations which took me way WAY longer than you’d expect from looking at the end result.
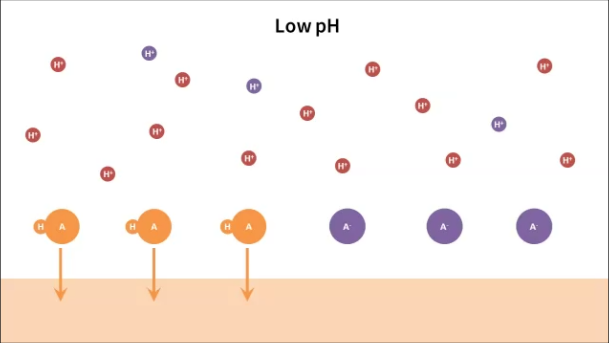
The video is on why pH matters for acids in skincare. I touch on why different acids need different pHs, why free acids penetrate better, and why some acids aren’t affected by pH, amongst other things. (Chemistry and pharmacology nerds out there, it’s very simplified… I should have talked about anion stability and not just bond strength, but cut me some slack!)
I know it’s not the best quality, but I wanted to get something out there to act as a starting point, and I kept changing my mind on what to use and I just reallyreallyreally needed to get it out of my hands so I’d stop fiddling with it. I’d love any feedback and tips you have!
The things I’m particularly annoyed about are:
- Sound quality – I’m recording on my phone’s voice recorder program, and I’m struggling through the back end of a cold after a 24-hour teaching week. The volume isn’t super consistent because I didn’t manage to record it all in one go, but I feel like there must be a video editing program that would let me fiddle with that?
- YouTube know-how – I have none. What have I forgotten to do? Hit me with all 5000 obvious things I should’ve done…
- My phlegmy throat. I’ve taken pseudoephedrine, Zyrtec, guaifenesin, bromhexine and warm honey water… what else can I shove in?
- My hair. Will my hair ever not be frizzy?
All that aside, here it is – my first video! Check it out here.
Please subscribe to my lonely little channel! And if you have any requests for video topics, let me know! Thank you for watching, and all those other things real YouTubers say ^_^ And if you hate videos, don’t worry – this blog will still be going.





I love the way you don’t waste our time with filler. So much great info in a crisp delivery. Learned so much!
Love it! Thanks for sharing your knowledge.
Great job, keep them coming
So thrilled to be an early subscriber to your YouTube channel. And don’t worry, the more you film the more comfortable you’ll become.
You’re my favourite blogger even though I’m on the other side of the world.??
And I love all your sciencey explanations.
This is awesome and so informative, thanks so much Michelle! Definitely keen for more!
Great video. Keen to see more.
It’s absolutely brilliant, I love it! And it’s nice to see you. I would love to see more skintellectual videos. ?
Loved it! To the point, simple and clear. The only suggestion is to speak a little slower and leave the graphics up a tad longer for those who are slower absorbing information. Truly you are a gifted teacher (I am also chemist but my calling was pharmaceutical research). I have a preference for written words in a blog (especially love all your diagrams) vs a vlog – primarily because I am more visual that auditory; second and third, it’s generational (I can’t abide rambling cutesy vloggers which is distracting) and I snag time during lunch at work and don’t want the office to hear the vlogger. I hope you continue to do both – being able to go back and re-read and study your diagrams is what draws me to your page! Thanks for bringing the science to us!
I agree with the point about the speed, I had no interest in (and thus no knowledge of) chemistry before my skincare addiction kicked in, so I need a little extra time to process everything. Taking the pace down just a tiny bit and leaving the illustrations up a little longer too would be of great help! Also, may I suggest: I wouldn’t mind you using an actual whiteboard or something if that helps you. Feels like school again, but in a good way.
Thank you! I’ll talk a bit slower next time, I think I was just nervous 🙂
I love the video, perfect length, please do more!
P.s.- I thought your hair looked great but since you were complaining about frizz, living proof no frizz shampoo has changed my life
Yay! Congrats on your first video! Very clear explanation, nice to hear your voice. 🙂
I love your voice!! The content is good too of course!
Thank you! 😀
What a treat to see you in action. I have been following you forever, and you are even more delightful than I imagined! I agree about the filler, too. I am retired teacher/librarian and found that kind delivery reaches EVERYONE of your students, no matter what their level of accomplishment may be. Also I suspect with your background and sense of whimsy you could do a marvelous video on how wrinkle or pimples actually form!
Oh yes…and after 60+ years of curly, frizzy hair I found that moisturize, moisturize, mositurize, is the answer. Have you heard of NaturallyCurly.com? Lots of wonderful hair science there!
Thank you so much! 🙂
I’ve been using lots of moisturising conditioners lately but I think my hair is just a bit slow to adjust 🙁 And yes, I love that site! So much good info!
Great video!!! Thank YOU!
Fantastic job! Very clear, concise and understandable. Would love to see more!
Have you tried hair oil on your ends? Helps me a lot with frizz on my bleached blond locks.
And I don´t think you really forgot anything major. Maybe introduce yourself a little more in detail and give some background on why you are so familiar with this stuff? For people that don’t read your blog.
I’ve been trying out hair oils but I think I forgot to use coconut oil as a prewash before this video! Thanks for the reminder 🙂
That’s a good idea! I’ll do that next time!
Just wanted to say great content, easy to understand, please slow down, straight to the point, no boring intro/ filler and I look forward to more videos! Thank you!
Would love a video on reading ingredient list.
Thank you! I’ll slow down a bit next time 🙂
Hi Michelle! I’ve been reading your blog for ages but never said hi. What a great video you made, you make it so easy to understand! Even though I can’t tell your frizz, me here also has chronic frizzy hair so I get you..
Thank you so much! 🙂
Extremely helpful and I’m so new to this skin care stuff so thank you!
Hi Michelle,
Super helpful and informative. Thank you Soooo much.
So I’m now considering your AHA guidelines when looking to purchase a product whose core ingredients are chemical exfoliants. Dermatologica has recently released its “Super Exofoliant” called Superfoliant but its pH is 7 and it does not report the percentage of AHAs in the product. You advise not to go over pH of 4, or it doens’t work cuz it doen’st penetrate the pores. But their claim is that part of the magic of their product is the opening of pores, to get at all thedirt and grime associated with air pollution, the new big enemy of great skin.
My questions are this: 1. Do you think the AHAs will work under these conditions and
2. What’s the likelihood a manufacturer will tell you the percentage (4-10% I believe you advise) of AHA and BHAs contained in their products? (So far I’ve received no replies….)
Appreciate your thoughts, thanks! Lisa
I actually reviewed the Dermalogica Superfoliant earlier! IMO the acids in there are a bonus that may or may not work, most of the exfoliation from that product is physical. In terms of percentage – brands seem to either be super forthcoming or not at all. I’d personally only splash out on a product with a known percentage, and I think more and more people are doing this now as well.
Hi Michelle,
It was a great and really helpful video!
The only think that I don’t get with the acid exfoliants is whether they are still effective in case they are dissolved in an oil product or not. As you said oils are non polared. So I wondered, what would the effect be: will the acid remain in a form that will not penetrate or the opposite and why? An example of this product is Bobbi Brown’s Remedie no75 Skin Clarifier which is an oil with salicylic acid.
Sorry for my bad english.
Thank you.
It’s difficult to say for sure, but for salicylic acid, it looks like it’ll penetrate in an oil base. Since oil is non-polar, the salicylic acid won’t ionise, so it’ll stay in free acid form. Once it interacts with the skin it might do something, but it should be more effective than water-based!
Thanks a lot!
Have you ever tried a product like this, I mean an oil with salicylic acid or lha in it? Also, I am a little bit confused on wether an acid is ionised in its natural form or it becomes ionised when it is dissolved in a polared medium.
Hi, I just read the post “The differences between BHA and AHA” . In that post, you said both BHA and AHA loosen the dead skin cell connection on the face surface so is it necessary to use both?
I wouldn’t say necessary, but I found that they have slightly different advantages for my skin.
What a clear and engaging video! I wonder if I can get help with a few clarifications…
– What are the hydrogen ions and the anions doing when they are unavailable as “free acid molecules”? Are they just swimming around in the solution/staying on top of the skin as ions and/or binding randomly with any other ion with the right charge?
– Since there is more oil than water on the surface layer of the skin, does it mean that anhydrous formulas (e.g., ascorbic acid in silicone base) are more effective than aqueous formulas? Does this depend on whether the ascorbic acid “powder” in the anhydrous base is oil-soluble (are they?)
– I also wonder what it means when we call an acid “effective” and “stable” (and the right pH is often proposed as the condition that makes both possible). Take ascorbic acid as e.g. again, as an anti-oxidant, it protects skin cells by oxidizing itself in place of the skin cells/proteins (right?). When we call a certain AA product stable, we often mean that it can stay unoxidized in the bottle/tube for a long time. But how does this “stable” molecule (defined by its resistance to oxidation) become “effective” (defined by its ability to oxidize itself) once it enters the skin? I’ve always thought that these “stable” forms are converted into charged forms in the skin before they become effective. But the video seems to suggest that charged forms are less effective than “free acid molecules” that are happily, stably, paired up, and uncharged? Do the molecules stay “wholesome” in skin cells and only oxidize themselves when oxidative stress strikes? I have serious knowledge gaps in my understanding of this complex chain of processes so please forgive my long-winded question!
On this note, would you consider doing a future video on how “anti-oxidants” work, and why they are sometimes oil, sometimes acids, may have exfoliating properties, and what these means for how we use them?
Many apologies for the wordy questions. Please feel free to respond as briefly as you have time for! Much appreciated!
>What are the hydrogen ions and the anions doing when they are unavailable as “free acid molecules”? Are they just swimming around in the solution/staying on top of the skin as ions and/or binding randomly with any other ion with the right charge?
They’re mostly just swimming around, if there’s any water present.
>Since there is more oil than water on the surface layer of the skin, does it mean that anhydrous formulas (e.g., ascorbic acid in silicone base) are more effective than aqueous formulas? Does this depend on whether the ascorbic acid “powder” in the anhydrous base is oil-soluble (are they?)
You’re right – oil-soluble things in oils would be the best. If it’s insoluble, some will penetrate but very little since most of it is in one big lump that can’t pass through skin.
>I also wonder what it means when we call an acid “effective” and “stable” (and the right pH is often proposed as the condition that makes both possible). Take ascorbic acid as e.g. again, as an anti-oxidant, it protects skin cells by oxidizing itself in place of the skin cells/proteins (right?). When we call a certain AA product stable, we often mean that it can stay unoxidized in the bottle/tube for a long time. But how does this “stable” molecule (defined by its resistance to oxidation) become “effective” (defined by its ability to oxidize itself) once it enters the skin? I’ve always thought that these “stable” forms are converted into charged forms in the skin before they become effective. But the video seems to suggest that charged forms are less effective than “free acid molecules” that are happily, stably, paired up, and uncharged? Do the molecules stay “wholesome” in skin cells and only oxidize themselves when oxidative stress strikes? I have serious knowledge gaps in my understanding of this complex chain of processes so please forgive my long-winded question!
Charge and stability are pretty different concepts – charged generally refers to if it can easily go back to its original uncharged form, while unstable means it’s changed into something else that can’t turn back into the original form (e.g. if irreversibly oxidised). Charged forms can’t get through skin. Hope that makes sense!
>On this note, would you consider doing a future video on how “anti-oxidants” work, and why they are sometimes oil, sometimes acids, may have exfoliating properties, and what these means for how we use them?
I’ll put it on the list! 😉
This is an old post, hope you’re still reading comments on them! This is a fantastic explanation of pH and AHAs. Now my burning question is whether we have to wait to layer on other products. There is advice going around to wait 30 min before putting on another product, otherwise it would deactivate the AHA because you’d be raising the pH. However, another chemist on instagram (Ava Perki) showed how it would take 500-600mL of water to raise the ph of 2g of The Ordinary’s glycolic acid from 3.7 to 4 (debunking a doctor that claimed damp skin or other skincare products would deactivate glycolic acid). But this post goes into deep detail about pH in AHAs and gives instruction to wait 15 min for Vitamin C, and 30 min for AHAs: https://www.essentialdayspa.com/forum/viewthread.php?tid=18360
So what’s the real deal??