If you’ve ever looked up sodium laureth sulfate (SLES) and sodium lauryl sulfate (SLS) in a clean beauty app, like me, you might be surprised to find that SLES is dirtier than SLS.
These apps are terrible and based on pseudoscience: SLS and SLES are both safe in your products. But if you’re a skincare nerd, you’ll know that SLS is considered harsher on your skin than SLES. So… what’s with these ratings?
Related post: Clean Beauty Is Wrong and Won’t Give Us Safer Products
There’s actually a really interesting story behind this, about an innovation that’s so useful it’s everywhere in skincare, haircare and beauty. But I think most people haven’t even heard of it!
So today I’m talking about the chemistry of ethoxylation, how it turned into this fearmongering, and why you’re probably going to hear a lot more on this topic this over the next couple of years.
The video (located here) is sponsored by Croda, a cosmetics ingredient supplier who make tons of the ingredients in your products (both ethoxylated and unethoxylated), but as always, all the ingredients for this post were supplied by me.

Ethoxylation
If you look on the backs of your products, you’ll see this in a lot of ingredient names: “eth”.
A lot of the time (not always), these three little letters means the ingredient is ethoxylated.
There’s sodium laureth sulfate of course, but there’s also:
- Anything that ends with “eth” and a number (laureth, beheneth, ceteth, glycereth)
- Isethionates
There are other ethoxylated ingredients too without the “eth” – for example, PEGs and polysorbates.
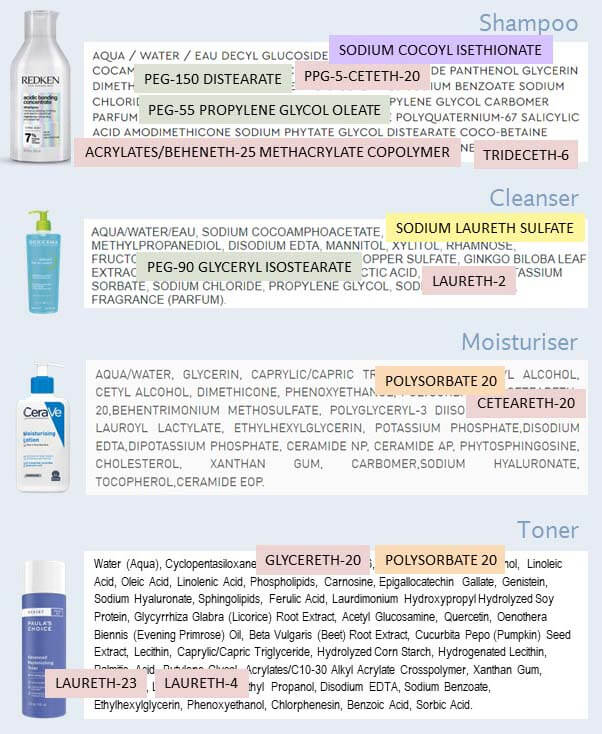
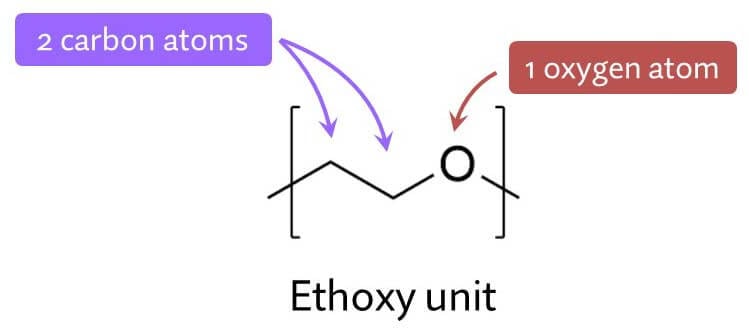
Ethoxylation means you take an ingredient, and you add little units containing two carbon atoms (which is what “eth” means in chemistry) and an oxygen atom (the oxy part).
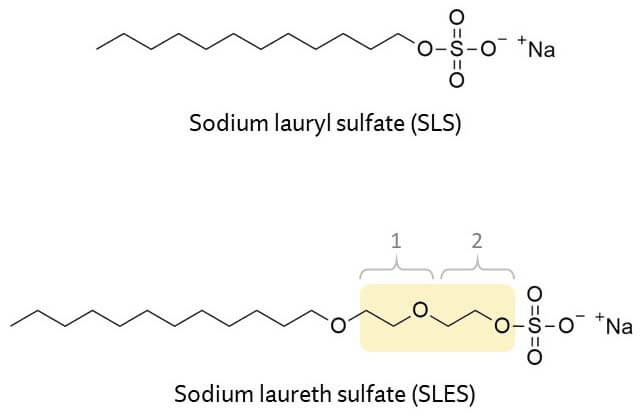
For example, here’s sodium lauryl sulfate and sodium laureth sulfate – you can see the extra added ethoxylated part. This particular sodium laureth sulfate molecule has two extra added ethoxy units.
These little 3-atom units are really important in changing the properties of an ingredient.
Most of these ethoxylated ingredients are surfactants. Surfactants have one end that likes oil and one end that likes water. They’re really important because, if you think about it, a lot of skincare and beauty is about trying to get oil and water to mix… which is the thing surfactants are best at!
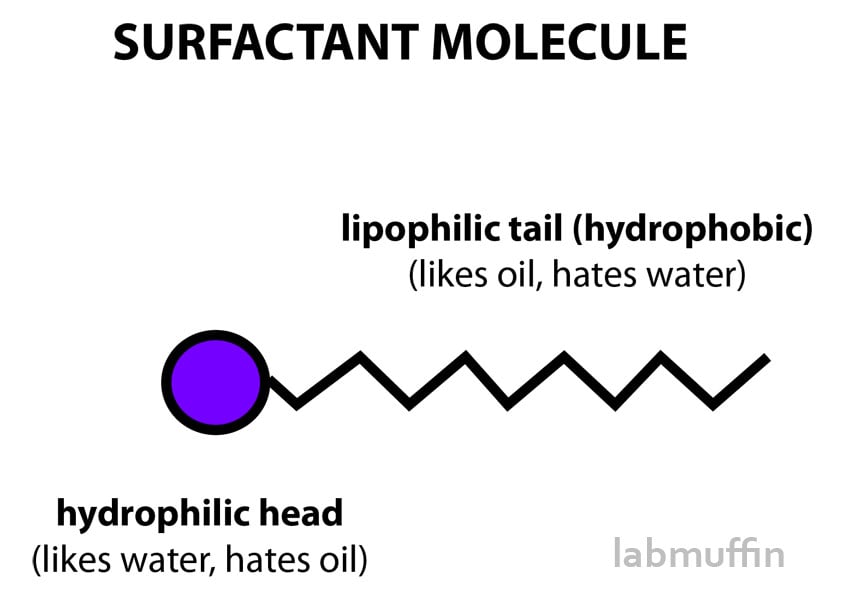
For example:
- When you’re cleansing your face or washing your hair, you’re trying to get oily substances to rinse off into water
- When you put on a moisturiser, you’re trying to add oil and water to your skin in the one product, so they have to behave together nicely
- A lot of fragrances are oily, but a lot of the time you have water-based products you might want to make scented
Surfactants are key to making all of these work.
The main reason ethoxylation is so useful is that it adds oxygen to the structure.
Oxygen is an element with special properties, and the main one is that it can form special bonds with water called hydrogen bonds.
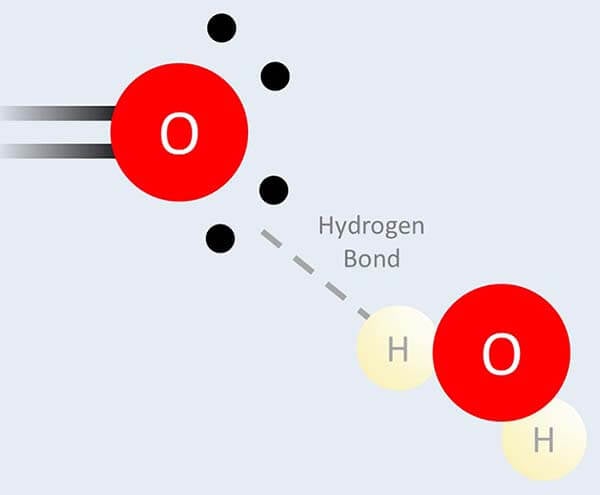
Ethoxylation makes an ingredient like water more and form more of these hydrogen bonds, and the more of these ethoxy units you add the more water-loving it’ll be. So it’s really handy for fine-tuning how much an ingredient prefers water versus oil, and therefore the specific properties of the final product.
The other big impact is that ethoxylation increases the size of the surfactant, and this means it doesn’t penetrate skin as easily, making the surfactant gentler on skin. This is why SLES is so much gentler than SLS. Even though they’re both sulfates, one study found that SLES could be gentler than SLS, as well as a whole bunch of non-sulfate surfactants.
Related post: Are You Washing Your Face Wrong? Busting Cleanser Myths (with video)
So thanks to ethoxylation, we can have:
- Cleansing surfactants that are gentle on skin, but still foam well
- Micellar waters with surfactants that cleanse skin well but are so gentle you don’t need to rinse
- Cleansing balms and oils that apply like an oil but rinse off cleanly
- Superfatting agents in cleansers, shampoos and conditioners that act like water-soluble oils, so your skin and hair can be moisturised while still rinsing clean
1,4-Dioxane
But here’s the catch: ethoxylation is linked with 1,4-dioxane.
1,4-Dioxane isn’t intentionally added to your beauty products – it doesn’t have a purpose in them. But it’s formed as a byproduct during ethoxylation, and ends up as a trace contaminant – it essentially hitches a ride with the ethoxylated ingredients that are intentionally added to the product.
During the ethoxylation process, you’re adding these little ethoxy units with 2 carbons and 1 oxygen to your ingredient. But sometimes, instead of reacting with the ingredient, two ethoxy units join together and you end up with this ring, 1,4-dioxane, as a byproduct.
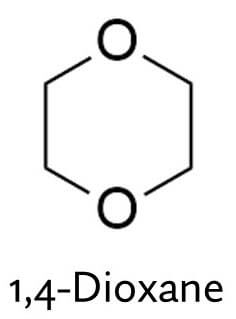
1,4-dioxane is not nice. The main concern is that it’s suspected to be a human carcinogen. Even though it hasn’t been properly linked to cancer in humans, the evidence that it causes cancer in animals is strong enough to believe it could happen in humans too, so it’s probably a good idea not to be exposed too much to it.
There are two ways we can potentially be exposed to 1,4-dioxane through our beauty products.
Firstly, when we use products containing traces of 1,4-dioxane. Most of the 1,4-dioxane evaporates into the air, but some of it can be absorbed through skin, and some of it might be inhaled.
Cleansing products like body wash and shampoo have the highest amounts of 1,4-dioxane (not surprising, since they have the highest amount of surfactants – surfactants are their active cleansing ingredients). There are also some differences between how different ingredients are made that changes how much 1,4-dioxane is in them. SLES usually has the highest levels of contamination because of how it’s synthesised.
But the levels of 1,4-dioxane in beauty products have actually dropped a lot over the years. Ingredient manufacturers have been working on reducing the amounts of 1,4-dioxane, without there actually being any laws in place. There are ways to produce ethoxylated ingredients with less 1,4-dioxane, and there are ways to remove a lot of the excess 1,4-dioxane afterwards, and these have been really effective.
The FDA has monitored how much 1,4-dioxane is in beauty products from the US over the years.
- In 1981 the average amount was 50 ppm, which is equivalent to one drop in a giant 1 litre bottle of shampoo.
- By 1997, the average amount had more than halved to 19 ppm, so a bit less than half a drop in 1 litre.
- In 2008, the FDA conducted another survey of 35 products – the highest level detected was 11.6 ppm, and 80% of products had levels below 1 ppm, which is less than one fiftieth of a drop in this bottle.
The amounts considered “safe” in beauty products are slightly different around the world. 10 ppm is the limit recommended by the scientific committee for consumer safety in the EU. This is the amount that they calculated would cause one 1 person in 7.5 million getting cancer per year, or the equivalent increased lifetime risk of cancer if you smoked half a cigarette per year for 60 years.
But like you saw, the vast majority of products don’t have this much anyway, so you don’t have to be all EWG about it.
New York has recently changed their law to limit 1,4-dioxane in cosmetics to 10 ppm by the end of 2022. But they’ve gone further with household cleaning and personal care products (defined as the ones you rinse off your skin): 1,4-dioxane in these will be limited to 2 ppm by the end of 2022, and 1 ppm by the end of 2023.
So why do the things that don’t stay on our skin have tighter limits?
Well, it’s because of the other, and overall bigger concern: 1,4-dioxane is a drinking water contaminant. And this is a very real problem. 1,4-dioxane absorbs easily from your digestive system, a lot of places do have quite high levels of dioxane in their drinking water, and most places don’t have laws on the allowed amount of 1,4-dioxane in drinking water.
The idea is: cosmetics stay on your body, but personal care products (which is defined as cleansing products including shampoo, conditioner, soap, bath products) go down the drain into the waterways, which eventually becomes drinking water.
So I guess limiting 1,4-dioxane in personal care products makes sense in theory, but I’m skeptical it’s going to do much.
1,4-Dioxane is a trace contaminant in personal care products, but it’s used intentionally in other industries. It’s a solvent that’s used in paints, inks, cleaning, and making plastics.
1,4-Dioxane was most commonly used as an additive to stabilise chlorinated solvents, which were phased out as part of the Montreal Protocol (also known as the one time the world got together and did something about an environmental disaster). The Montreal Protocol banned the use of substances that destroyed the ozone layer. The main chlorinated solvents that 1,4-dioxane was used with were banned in 1996. So the use of 1,4-dioxane has actually dropped massively from its peak in the 80s. In the US it’s dropped to about 1/25th of the peak, but about 1 million pounds (450 000 kg if you’re in a country that uses real numbers) of dioxane is used every year, and in 2016, 62000 pounds (28080 kg) of that was released into water.
So we can do a calculation to work out how much shower gel and shampoo you’d have to use to release the same amount of dioxane into the water:
- 28080 kg released to surface waters by US industry in 2016
- 2 ppm = 2 mg/L
- 28080 kg ÷ 2 mg/L = 1.404 x 1010 L
- 2016 US population = 323.1 x 106
- (1.404 x 1010) ÷ (323.1 x 106) = 43.5 L per person/yr
- 43.5 ÷ 365 = 0.119 L per day
If your products have an average of 2 ppm dioxane, every single person in the US would have to rinse 43.5 L of beauty products down the drain every year. That’s about 1/3 of this mug (119 mL), or 24 palm sized dollops of product every day.

And even then, these amounts currently released by industry every year aren’t thought to be the biggest contributor to 1,4-dioxane in drinking water either. The amounts used decades ago, before the chlorinated solvents were phased out, were much much higher. There are lots of old industrial areas and improperly lined disposal sites that are contaminated with 1,4-dioxane, and this can leach into underground water, which is where a lot of drinking water comes from.
A 2017 study found that dioxane in contaminated water was correlated with chlorinated solvent levels, which points towards these old industrial uses as the main culprit.

In New York, where this law’s being introduced supposedly to improve drinking water, it’s Long Island that has particularly contaminated water, and it’s thought that it’s because there are a lot of old industrial sites there, and their drinking water comes from underground aquifers. So to me, it seems like a much better approach would be to treat the industrial sites.
Either way, these new limits are coming in at the end of 2022 in New York.
What does this ban tell us?
So I think this is a really interesting story that highlights a lot of themes that come up a lot in tHe BeAuTy DiScOuRsE…
Theme 1: Really amazing innovations can become a victim of their own success – things that get popular get scrutinised, and if there’s any sort of fearmongering that can be done, you know that certain sections of the beauty industry are going to try to take advantage of it (cough clean beauty).
Crystal ball time – I think we’ll probably see some 1,4-dioxane scare stories around the end of the year when these restrictions take effect, a great Christmas present for me. I also think other places will make similar changes as well. Because…
Theme 2: Sometimes the easy things get regulated, and the hard but more important things get ignored. Sometimes maybe lawmakers genuinely don’t understand the issue, but sometimes this can be a strategic decision to look like they’re “doing something” – it acts as a smokescreen for inaction. Setting a limit that manufacturers have to meet is cheap and easy, but remediating industrial sites is a lot harder.
New York is going to evaluate whether this law actually worked in 2025, and I think they’ll say it did, because 1,4-dioxane levels have actually been going down anyway.

Plus, scientists are developing lots of ways of getting dioxane out of wastewater and drinking water. (I hope someone will do an analysis specifically to see if the personal care product limit makes a difference because I could be wrong…)
Theme 3: The beauty industry does proactively do good things without being forced to by specific regulations – we can see this in the fact that the US and EU have very similar beauty products containing very similar ingredients, even though clean beauty advocates will tell you that the EU has much stricter regulations that the US should emulate.
The catch is that a lot of it depends on what consumers want, so we need to be asking for the right things, not things that are based on misunderstandings of what the real issues are.
The video is sponsored by Croda, although the blog post is not. For more information, see Disclosure Policy.
References
H.P. Ciuchta, Dodd KT. The Determination of the Irritancy Potential of Surfactants Using Various Methods of Assessment. Drug and Chemical Toxicology. 1978;1(3):305-324. doi:10.3109/01480547809105022
14d. 14d. Itrcweb.org. Published 2016. Accessed May 3, 2022. https://14d-1.itrcweb.org/#gsc.tab=0
Adamson DT, E. Piña, Cartwright AE, et al. 1,4-Dioxane drinking water occurrence data from the third unregulated contaminant monitoring rule. Science of The Total Environment. 2017;596-597:236-245. doi:10.1016/j.scitotenv.2017.04.085






Outstanding breakdown and video! Thank you!!
Very interesting and presented in laywomen’s terms. Thank you.
Thank you for explaining this, I wasn’t aware of any of it.
Thank you for touching on this subject and going through some of the chemistry and details. You bring up really great points on the the lack of oversight for higher 1,4 dioxane polluting industries. Reducing our exposure to this non-degradable carcinogen should entail a multi-factorial effort: from remediation of polluted ground/water to preventing its deposition in the first place. Each polluting channel should be scrutinized and limited – from the industrial sector to home and personal care use – every bit counts because this compound accumulates. To say its not important for us to care about in our everyday cleansing is like saying I shouldn’t recycle because my plastic garbage is so much less than the industrial garbage that goes into landfill. If we don’t do our part, things certainly wont change for the better.
I agree the contribution of personal care products should be assessed, but focusing on a relatively insignificant issue often acts as a smokescreen for inaction on bigger issues unfortunately – for example, the NY ban in personal care products was announced with a lot of fanfare, but there hasn’t been any announcement for remediation of old industrial sites. This has been the case with plastic waste as well – the largest manufacturers of plastic waste are big financial supporters of cleanup efforts that aren’t likely to make much of an impact compared to reducing production…
Agreed, smokescreeens stand in the way of meaningful action. A multifactorial approach is needed from source to endpoint. I wasn’t aware of the lack of remediation efforts in NY. As a Brooklyner, i am going to actively look into this and see what I can mobilize on the local level. Thanks again for the thoughtful discussion. <3