I recently posted an excerpt from The Lab Muffin Guide to Basic Skincare on my Instagram and Facebook (which is one of many useful tables in my eBook by the way, and it’s packed full of information on the super important fundamentals of science-based skincare – although I’m obviously biased since I wrote it and put all my best tips in there!). Since then, I’ve had a lot of questions about whether X ingredient is a good humectant, or some other ingredient is a good occlusive, and how to tell if something is an occlusive or an emollient.
Unfortunately it isn’t always easy to tell what an ingredient will do. A lot of it depends on the formulation – a theme I talk about quite often. A small amount of an ingredient may be emollient, but a larger amount might also be occlusive. It depends on how the ingredient assembles itself on and in your skin.
But humectants are relatively easy to pick out, and it’s all about chemistry, so ya girl is going to give you the superpower of Picking Out (likely) Humectants right here! Hang on to your hats.
What is a humectant?
Quick reminder: a humectant moisturiser is an ingredient that sits on your skin and grabs onto water, slowing it from evaporating. This keeps your skin hydrated.
Polarity and intermolecular forces
I’ve mentioned before in my posts about surfactants, chemicals exist in two worlds: polar and non-polar.
- Polar roughly means water-loving, while non-polar roughly means oil-loving.
- Polar substances like hanging out with other polar substances, non-polar substances like hanging out with other non-polar substances.
What makes chemical substances like hanging out with each other, though? It’s something called intermolecular forces. These are tiny sticky interactions that substances can have with each other.
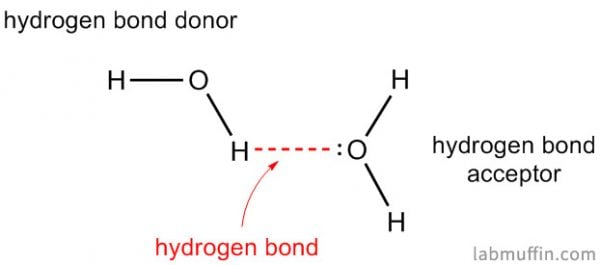
Water is special because it can form an especially strong intermolecular force known as hydrogen bonding.
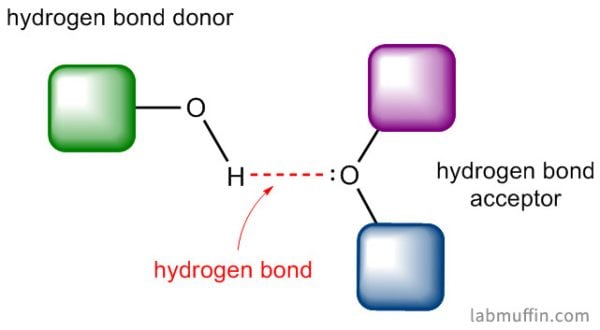
Hydrogen bonding ability
A hydrogen bond is a bit of a misnomer – it isn’t really a chemical bond, but it does involve hydrogen. What you need for a hydrogen bond to form are two things:
- A hydrogen bond donor: a hydrogen atom covalently bonded to a nitrogen, oxygen or fluorine atom
- A hydrogen bond acceptor: a lone electron pair on a nitrogen, oxygen or fluorine atom
Water is H2O, so it has both of these things.
If you want an ingredient that sticks to water and slows it from evaporating (i.e. acts as a humectant), you’re going to also need something else that can form hydrogen bonds with water.
In skincare, we generally don’t find fluorine, so we’re looking for OH and NH groups on our humectant ingredients.
Overall polarity
But that’s not quite enough – the overall molecule also needs to be polar enough to attract water.
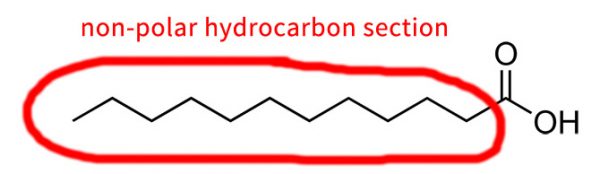
Hydrocarbon portions of a molecule – that’s bits that are made of hydrogen and carbon only – are non-polar, so they repel water. Sometimes carbons are represented using the symbol “C”, but usually in molecular structures it’s just a corner or the end of a line.
There needs to be enough NH and OH groups on the molecule to counteracts the non-polar parts, and end up with something that that sticks to water. You can think of the hydrocarbon parts and the OH/NH groups as non-polar and polar groups battling to change the overall molecule’s behaviour.
It’s a bit hard to say for sure how much of each you need, since the structure of the overall ingredient needs to be taken into account as well. As a general rule, somewhere around 1 OH or NH per 2-3 carbon atoms seems to work quite well.
Non-volatility
The final consideration for a humectant ingredient is that it won’t evaporate from your skin – in more technical terms, it needs to be relatively non-volatile.
Some ingredients that can hydrogen bond with water can actually dehydrate your skin by binding with water and taking the water molecules with it when it evaporates! Some ingredients that do this include ethanol and propanol, which is why they’re commonly called “drying alcohols”.
In general, if it has a high boiling point, it’ll stay on your skin (for more nerdy info about this sort of behaviour, you can read up on azeotropes). And very generally, the larger the ingredient and the more hydrogen bonding groups it has, the less volatile it is. Most hydrogen-bonding substances are non-volatile.
Humectant examples
Now that you know what to look for, let’s have a look at some ingredient structures so you can see how these considerations play out.
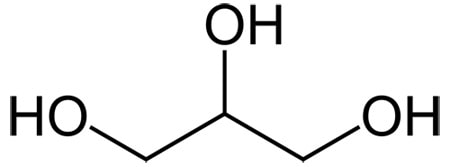
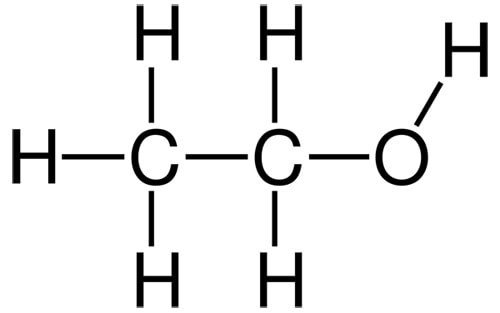
Glycerin
Glycerin is one of the best humectant ingredients – it has three OH groups for sticking to water, and only three carbons. Even though it’s small, it’s not volatile due to the OH groups sticking glycerin molecules together (it has a boiling point of 290 °C).
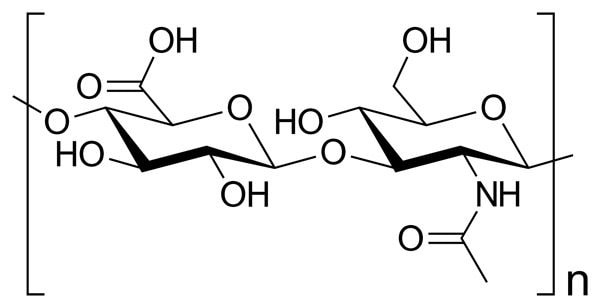
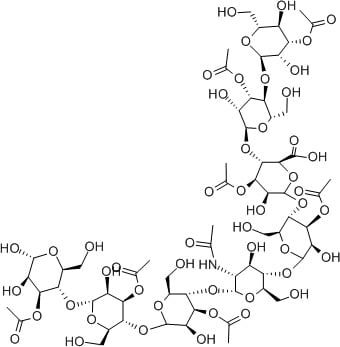
Hyaluronic acid
Hyaluronic acid is another fantastic humectant. Its structure is made up of units very similar to sugar, polymerised into long chains. There are heaps of OH groups everywhere.
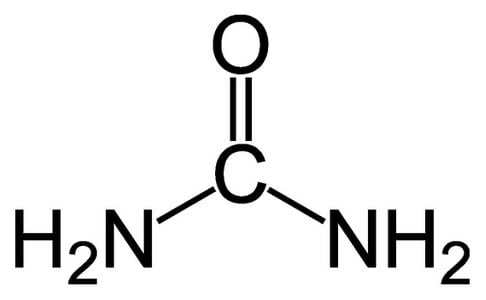
Urea
Urea is a bit unusual since it has NH2 groups instead of the usual OH groups.
Amino acids, peptides, hydrolysed proteins and proteins
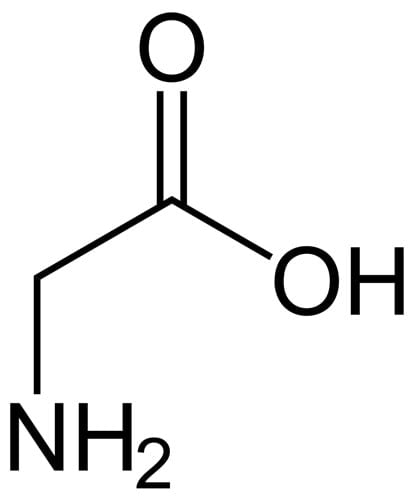
Amino acids contain an amine (NH2) group and a carboxylic acid (COOH) group, separated by a carbon atom. That means they’ll have both the NH and OH groups that you need for humectant action. Some examples in skincare: glycine, leucine, lysine, and arginine.
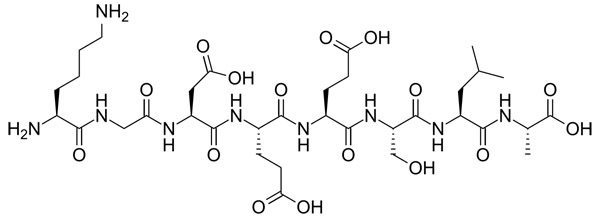
Peptides and proteins are chains of amino acids joined together, so again we have NH and OH groups. Hydrolysed proteins are proteins that have been broken up.
Aloe vera
There are a range of humectant substances in aloe vera, often called acemannan. Loads of OH groups!
Non-Humectant Ingredients
Now some ingredients that aren’t humectants:
Ethanol
Ethanol ticks two boxes for being humectant (hydrogen bonding, polar) but it’s volatile due to its small size, so instead of being a humectant, it binds to water then runs off with it. This means it will dehydrate your skin!
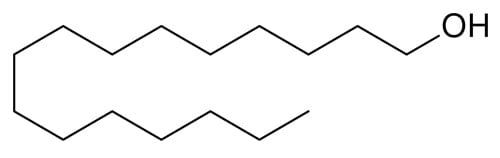
Cetyl alcohol
Cetyl alcohol can hydrogen bond (it has an OH group) and it’s non-volatile, but it’s too non-polar to interact with water effectively due to its long hydrocarbon chain. It’s an emollient moisturiser instead.
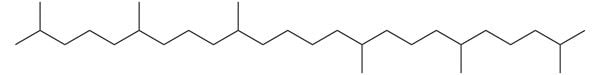
Squalane
From squalane’s structure, you can see that it has no hydrogen bonding groups, so there’s no way it’s polar enough to go near water. It’s an emollient.
Silicones
There are a range of silicones. Dimethicone is shown above – it has oxygen, but not really enough to bind to water properly. You need that extra hydrogen on the oxygen to have an effective humectant.
So I hope I’ve convinced you that structural analysis is pretty cool! But it’s worth keeping in mind that this isn’t the end of the story. There are also additional considerations beyond simply structure that can change how well it works as a moisturiser. For example, glycerin can pass through deeper into the skin than usual due to its ability to be transported by aquaporins. Urea in higher amounts can also exfoliate skin, which softens it too. Some metal ions can also act as humectants, since they bind water in a different way (through coordinate bonds).
Some humectant products:
The Ordinary 2% Hyaluronic Acid + B5
Indeed Labs Hydraluron Moisture Booster
Drunk Elephant B-Hydra Intensive Hydration Gel
Klairs Supple Preparation Unscented Toner
More about picking a suitable moisturiser and using it in your routine: The Lab Muffin Guide to Basic Skincare
This post contains affiliate links – if you decide to click through and support Lab Muffin financially, thank you! For more information, see Disclosure Policy.







I thougt humectants are drawing water from the deeper layers of the skin, and therefore they shouldn’t be used without occlusives which prevent water from evaporating?
Humectants will hold onto any water they bump into, whether it’s water from the product it was in, or water that’s going past as part of TEWL / osmosis, which would be from the lower layers. Whether they work well without occlusives is up for debate, but there’s evidence that glycerin by itself will increase skin hydration over time without an additional occlusive. They’ll work better with occlusives though!
If humectants prevent water from evaporating, why do we need occlusives to prevent TEWL? Usually when you use a hydrating toner with just humectants you lose that hydration pretty quickly unless you apply a moisturizer too.
No moisturiser can completely prevent water from evaporating, not even occlusives. All of them can only slow them from evaporating, which is why a combination of moisturising ingredients usually work best for most people.
Structural analysis used that way is absolutely frigging cool!
Anne|Linda, Libra, Loca
For me I find urea as being one of the best of ingredients that can moisture my skin, better than hyaluronic acid, ceramide, etc.
I always read and enjoy your posts tremendously. However, I never leave a comment because I end up speechless due to your vast knowledge and passion about all skin-related. I would really appreciate you wrote a post on rosacea, and current treatments. I’ve read some derms are using retinoids, but I’d like to know the evidence behind it. Thank you for spreading the word of the science. Xxx. Lucía