You might remember a similar post that I published in May 2019, after the previous flurry of “The FDA Admits Sunscreens Are Poison” headlines. Now that the second study has been published, it’s time for Round 2.

Gratifyingly, this time the coverage seems a little less hysterical (maybe they learned from last time?), with the exception of the Daily Mail, and media outlets that approached people who… weren’t really relevant experts. A lot of them seem to have forgotten that the study last year already found that sunscreens enter the blood:



Again, let’s look past the headlines at what the study tells us, and what this means for sunscreens and our health.
Why are these studies being performed?
In 2019, the FDA proposed a new rule about how sunscreens should be regulated. As part of this, the FDA decided they needed more data about the absorption of some sunscreen ingredients through skin before being able to recognise them as “generally recognised as safe and effective” (GRASE).
What was the first study about?
The first study, performed by FDA scientists and published in May 2019, simply showed that 4 active ingredients in sunscreens (avobenzone, oxybenzone, octocrylene, and ecamsule) were absorbed into the bloodstream and reached levels higher than 0.5 nanogram per millilitre (ng/mL) – the concentration at which the FDA considers ingredients safe by default.
The study looked at maximal use conditions (i.e. much higher than people would normally use): 24 volunteers applied 4 different sunscreens at 2 mg/cm2 on 75% of their body every two hours, 4 times each day for 4 days (16 total applications).
While this study was mostly meant to be a test run for the second study, there were some interesting findings:
- Blood plasma levels exceeded 0.5 ng/mL after 4 applications on Day 1 for all four sunscreen ingredients.
- The average maximum plasma concentrations for each sunscreen during the 4 days of the study were determined (1.8-4.3 ng/mL for avobenzone, 169.3-209.6 ng/mL for oxybenzone, 2.9-7.8 ng/mL for octocrylene, 1.5 ng/mL for ecamsule)
- The sunscreen ingredients remained detectable in blood for at least 3 days after the last application
What’s new in the second study?
The new study, published by the same research group in January 2020, is different in a few ways:
- 4 sunscreens containing 6 active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, octinoxate) were studied, rather than 4 active ingredients
- More participants (48 rather than 24 subjects)
- Blood concentrations were monitored for longer after the study was finished
- Skin concentrations were also measured
The biggest difference is that this second study didn’t just focus on maximal use conditions – it also looked at the plasma concentration of sunscreen ingredients after a single application:
- On Day 1, sunscreen was only applied once (2 mg/cm2 to 75% of the body) (single application)
- On Days 2-4, sunscreen was applied like in the first study (4 times a day every 2 hours) (maximal usage)
The results:
- All of the tested sunscreen ingredients exceeded 0.5 ng/mL in most participants after a single application. The maximum plasma concentrations after a single application were 0.7-1.6 ng/mL for avobenzone and 85.4-94.2 ng/mL for oxybenzone)
- The average maximum plasma concentrations for each sunscreen ingredient during the 4 days of the study were determined (3.3-7.1 ng/mL for avobenzone, 180.1-258.1 ng/mL for oxybenzone, 6.6-7.8 ng/mL for octocrylene, 13.9-23.1 ng/mL for homosalate, 4.6-5.8 ng/mL for octisalate, 5.2-7.9 ng/mL for octinoxate)
- Sunscreen ingredients remained over 0.5 ng/mL in the blood for different amounts of time after the study – unsurprisingly, the ones that reached the highest concentrations also took the longest to leave (In more than 50% of participants, plasma concentrations remained above 0.5 ng/mL for up to 7 days with avobenzone, octisalate and octinoxate, for up to 10 days with octocrylene, and for up to 21 days for homosalate and oxybenzone)
- Sunscreen ingredients stayed on/in the top layers of skin for quite a long time, suggesting that sunscreen is absorbed through skin quite slowly
But what does this mean?
This second study is far more relevant to everyday sunscreen use than the initial maximal usage study. While the single application is hefty (most people apply around half of the recommended amount, and 75% of your body is a lot), the blood concentrations are probably achievable by most people on a beach day after a few applications of sunscreen.
But despite the increased relevance, if you’ve been reading my blog for a while, you’ll know the important caveats about finding stuff in your body:
- Just because something is there doesn’t mean it’s doing harm
- Even if it’s been found to be harmful in cell and animal studies, humans aren’t cells or animals, and putting something on your skin is not the same as eating or injecting it (a point that the EWG and media outlets like the Daily Mail seem to purposely ignore)
Absorption does not mean harm
Let’s look more closely at the significance of “0.5 ng/mL”. A lot of media outlets are reporting this is “the FDA’s safety limit”, but this is inaccurate – it’s the concentration for which the FDA considers a sunscreen ingredient safe by default, according to the 2019 proposed rule. It’s low enough that the chance of cancer developing for any substance would be lower than 1 in 100,000 after a single dose, so the logic is that it’s low enough that no other safety data is required (although it’s been argued that this threshold should be even lower).
In other words, finding that the ingredients get into blood at a higher concentration doesn’t mean that they’re no longer safe – it just means the FDA requires more data to be submitted before they can say that it’s safe under the new proposed rule.
Like last time, the FDA press release accompanying this study that everyone continues to ignore says:
“Without further testing, the FDA does not know what levels of absorption can be considered safe.”
The FDA press release also highlights that
“Absorption does NOT equal risk – The FDA advises continued use of sunscreens
The findings in these studies do not mean that the FDA has concluded that any of the ingredients tested are unsafe for use in sunscreens, nor does the FDA seeking further information indicate such. The agency’s proposed rule requested additional safety studies to fill in the current data gaps for these ingredients.
Given the recognized public health benefits of sunscreen use, the FDA strongly advises all Americans to continue to use sunscreens in conjunction with other sun protective measures (such as protective clothing)”
The study points this out as well:


And again, most media outlets have decided to lean into the potential harms of sunscreen ingredients instead – clickbait pays, journalistic responsibility be damned.
Should you switch from chemical to mineral sunscreens?
All this uncertainty and lack of data sounds pretty scary, so it’s tempting to think that switching to mineral sunscreens (which have already been categorised as GRASE by the FDA) is the clear solution.
Everyone has different risk factors to take into account, and the risk calculus will be different for different people, as the editorial accompanying the article mentions. (There’s also a tweetorial from one of the JAMA Dermatology editors commenting on the study.)
Some relevant points to consider:
There’s still no real-world evidence that sunscreens are harmful to humans
Oxybenzone has been used in US sunscreens since 1978, and exposure is ubiquitous – yet there haven’t been any long-term harmful effects found (beyond limited effects like rashes and allergic reactions). Oxybenzone is the sunscreen ingredient that seems to be the most risky in terms of potential endocrine and reproductive effects, so it’s likely that the others will have even less of an effect.
On the other hand, there’s much clearer evidence linking excessive sun exposure to skin cancers, and sunscreen to skin cancer prevention. A sunscreen is only protective if you apply it regularly, in adequate amounts, so the well-known drawbacks of mineral sunscreens (like white cast) could potentially cause underapplication, which means you trade the potential harm of chemical sunscreens for the real harms of excessive sun exposure.
Different populations will have different risks
The vast majority of studies on skin cancer and sun exposure are on white adults, so the benefits aren’t necessarily generalisable to other people. As Adamson and Shinkai point out in their JAMA editorial on this study, the cost-benefit calculus of wearing sunscreen will be different depending on your individual circumstances.
- Skin colour changes sun exposure risks – most advice about sunscreen and skin cancer is intended for a white population. Sunscreen use will be much more beneficial for a white person in Australia than a black person in the UK, for example, so the potential benefits of sunscreen application will decrease relative to the potential harms.
- Children likely have increased absorption of sunscreen ingredients (their body surface area to volume ratio is higher), and any potential health harms from sunscreens will probably have a higher impact. However, a disproportionate amount of UV exposure occurs in childhood, which can lead to skin cancer later in life.
- The frequency and quantity of sunscreen applied will also change the relative risks.
It’s best to talk to a medical professional if you’re unsure of what the best option is for you. As well as mineral sunscreens, the JAMA editorial points out that clothing, sunglasses and avoiding intense sun exposure are also important.
It’s also worth noting that the US hasn’t approved the newer chemical sunscreen filters yet, which usually penetrate skin less – JAMA is a US journal, so the editorial advice may be more biased towards mineral sunscreens (as is often the case with advice from US dermatologists).
It’s also worth noting that if you’re a skincare fan who uses anti-aging ingredients – daily sunscreen is probably a good idea, since these ingredients can increase your susceptibility to sun damage.
Related post: Sun Protection and Vitamin D Deficiency
Other regions regulate sunscreen differently
The main reason these studies have been performed is because the FDA has changed how it wants to regulate sunscreens – essentially, it no longer assumes that sunscreens aren’t absorbed through skin. But other places – like the European Union – have been regulating sunscreens differently for years, taking absorption through skin into account.
Again, as I mentioned last time, similar concentrations of sunscreen ingredients have been reported in blood and urine before, so while the new FDA study gives additional and corroborating information, it doesn’t drastically change the current state of knowledge on sunscreen safety.
Here’s a comparison of the current limits for the 6 ingredients in this study in the US and EU:
- Avobenzone
- US: 3%
- EU: 5%
- Oxybenzone
- US: 6%
- EU: 6%
- Octocrylene
- US: 10%
- EU: 10%
- Homosalate
- US: 15%
- EU: 10%
- Octisalate
- US: 5%
- EU: 5%
- Octinoxate
- US: 7.5%
- EU: 10%
As you can see, the US limits are already pretty similar to the EU limits (and in some cases lower), so practically speaking, the vast majority of US sunscreens do already follow regulations that take into account absorption of sunscreen ingredients (even though there are still gaps in the data – which will hopefully be filled faster now that the FDA’s position has changed).
Maybe good news for newer filters?
Newer organic sunscreen filters are often designed to be harder to absorb. Their structures are generally larger than the older organic sunscreen filters in this study, and smaller things get through skin more easily, sort of like when you try to make yourself streamlined to go through a crowd quickly.
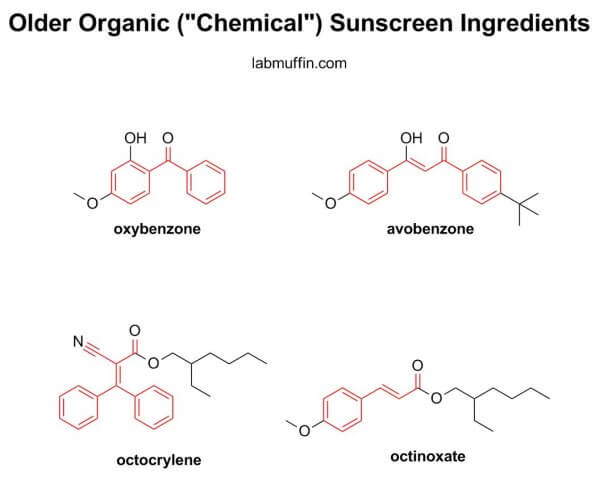
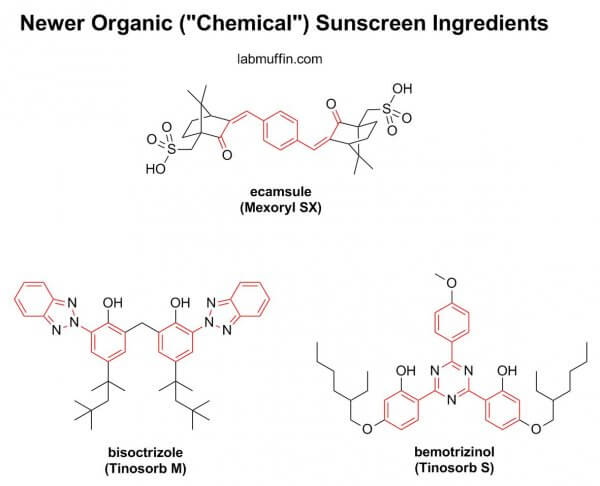
Perhaps this is overly optimistic of me, but I’m hoping that this study highlights to the FDA the need to fasttrack the approval of the newer filters, which have been used in other parts of the world for many years already.
What percentage of applied sunscreen is absorbed?
There was a common misinterpretation of the first study’s abstract:
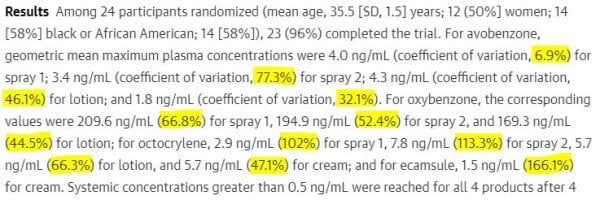
Many people assumed that the percentages listed after each filter was the percentage of the filter absorbed. This isn’t the case – it’s the coefficient of variation, which measures how different the measurements from different participants was.
From the plasma concentrations and the study protocol, we can make a rough estimate of the actual concentration of the applied avobenzone that was absorbed into the blood at that point in time (thanks to /u/nerd281 for allowing me to present her calculations!)
Average body area of 1.7 m2 = 17000 cm2
Sunscreen applied to 75% of body at 2 mg/cm2
Total mass of sunscreen applied = 34000 mg
3% avobenzone in sunscreen
Total mass of avobenzone applied = 1020 mg
4 ng/mL detected in plasma
Plasma is 58% of blood
Average blood volume = 5 L = 5000 mL
Total avobenzone absorbed = 4 x 0.58 x 5000 = 11600 ng = 0.0116 mg
Percentage of avobenzone applied that was absorbed into the bloodstream for this concentration = 0.0116 / 1020 x 100 = 0.0011% absorbed
This is very very different from the coefficients of variation in the abstract: 6.9-77.3%, which is between 6900 and 70000 times higher! (Note: this percentage won’t be all the avobenzone that’s absorbed, since it’s leaving the blood and being excreted too.)
Reading peer reviewed scientific papers often isn’t as straightforward as it may seem – there are lots of things that scientists find important to present that consumers don’t ever need to consider, and information that consumers find important that scientists don’t see the need to talk about in the context of the study.
Related post: The “60% of products absorb into your bloodstream” myth
References
The new study: Matta MK et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial, JAMA 2020, 323, 256–267. DOI: 10.1001/jama.2019.20747
Editorial on the new study: Adamson AS & Shinkai K, Systemic absorption of sunscreen: balancing benefits with unknown harms, JAMA 2020, 323, 223-224. DOI: 10.1001/jama.2019.20143
Tweetorial on the new study from @AdeAdamson, 22 Jan 2020:
Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: https://t.co/uzbaQpa3jr PART-2 of a study on chemical sunscreen absorbing into the blood. This is surely going to stir up more controversy. Let me break it down in this thread #tweetorial 👇🏾
— Ade Adamson, MD MPP (@AdeAdamson) January 21, 2020
The old study: Matta MK et al., Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial (open access), JAMA 2019, 21, 2082–2091. DOI: 10.1001/jama.2019.5586
My blog post on the old study: Sunscreens in your blood??! That FDA study (9 May 2019)
Commentary on the old study: Charalambides M et al., Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a critical appraisal, Br J Dermatol. 2019 Dec 13. DOI: 10.1111/bjd.18803
American Academy of Dermatology, Update on photoprotection: What dermatologists need to know, Dermatology World Meeting News, 25 Jul 2019.





Thanks for going over the information. It nice to have some one be a voice of reason and not be freaking out. You post are great reads and worth sharing.
This is such a great blog post! So informative with plenty of links to primary sources. Thanks for demystifying it all and not jumping on the fearmongering train!
Thanks so much for this post, Michelle! You rock ?
Very good post, however I’m not willing to accept that “we don’t know” if these chemicals are harmful when absorbed into the bloodstream, since it hasn’t been proven or disproven by science. There have been far too many cases of harm being done after good science has either not been done at all, or has been buried, because to reveal it would negatively impact profits.
In America profits rule over human health, and this is getting worse by the day, not better.
Not sure the FDA profits off of us dying from cancer from not using sunscreen.
In the US, we just assume the FDA is trying to kill us out of sadism, not profit. Lol.
Exactly.
Smoking wasn’t considered harmful either, until it was…
There are also plenty of things that were considered harmful that turned out to be fine – electricity, masturbation, women voting…
These sunscreens are also approved in the EU.
so glad I have used mineral sunscreens for many years…though find European sunscreens interesting…
re: the section on newer filters, i thought ecamsule was one of the 4 actives in the first study and therefore shown to have been absorbed into the bloodstream?
Thank you – this is fascinating, interesting, well written and really helpful. I’m a cosmetic tattooist and also offer micro-needling and basic skincare to my clients. I’ve done some basic training in recognising signs of possible skin cancer, how to raise concerns with clients and how to refer on. I’m always reminding everyone to use sun cream. This article has really helped me to understand the differences in sun creams and their risks so I can respond to questions from my clients.
This is so ridiculously good to read! It explains so much behind the science. Breaks the data down so that the average person can understand. I usually use a mineral sunscreen. My biggest problem is getting a white stain on the inside collar of my dark blue work uniform shirts, and how to get those stains out. But regardless, I still put sunscreen on every morning. And try to reapply by midday.
I absolutely don´t get why some media seem to be on a mission to reduce sunscreen use – the link between UV exposure and skin cancer is there and well established, but that somehow doesn´t seem to count.
Hello, this my first comment. Thank you for the article. English is not my mother tounge, so maybe you wrote this and I didn’t get it – but my question is: Have Tinosorb and Mexoryl been tested in that way?
Hi Michelle!
This post eased a lot of my sunscreen worries!
I had another general question about sunscreen application. I’m using Hylamide’s HA Blur in my routine which is a mix between a mattifying primer and a moisturizer and don’t know whether to apply it before or after sunscreen. I know moisturizer is supposed to go before sunscreen and primer after from one of your other posts but don’t know what you should do when the product is both??
Thanks in advance!
Jonathan
Excellent information as always.
In the US the FDA has a very short list of banned substances in skincare (about 11) whereas Europe has banned over 1300!
Check out Dr YOUN for a very good YouTube video on the subject here: https://youtu.be/vKmSiPMNal8
My dermatologist recently told me NOT to apply sunscreen above my eyes or near my hairline or eyebrows as it can cause the hair to fall out (apolegia?)(sp)
Unfortunately Dr Youn seems to be peddling a lot of the myths that the Clean Beauty movement promotes. I’ve talked about clean beauty here: https://labmuffin.com/clean-beauty-is-wrong-and-wont-give-us-safer-products/
This is refreshingly sane and well-researched. A rare treat to read.
Here’s the deal with the FDA: they’re a handful. For example, on the one hand, they don’t approve certain over-the-counter supplements that are backed by scientific data and that psychiatrists might give to people suffering certain disorders.
On the other hand, they frequently approve of prescription-only drugs that CAUSE cancer. So much do they cause cancer, that advertisements for them have to warn “causes rapid-onset lymphoma.” FDA approved.
Obviously in Australia, ya’ll aren’t getting our TV commcerials for drugs (yes, it’s still legal to advertise prescription meds on TV, lots of people want it overturned but there’s a lot of obstruction in the gov’t) but we have ads for drugs that literally declare side-effects like neurological disorders and sudden death.
It sounds like an American exaggeration of hysterics, but it’s not — one of the most often advertised drugs on TV is Humira.
https://www.humira.com/
They advertise it for everything from skin disorders to severe arthritis. It does work for arthritis — it works by shutting off your immune system.
In a friendly voice, the advertisement warns that you can’t visit places with large crowds or get near anyone sick. (Which is physically impossible.)
It is a drug that kills people, but because they’re upfront about the warnings, the Humira people can’t get sued.
If you ever come to the US and see people in SARS-like surgical masks, it’s because they’re on Humira.
This drug is advertised multiple times a night during various commercial breaks.
It call comes back to the question of, WHY would the FDA approve a drug that kills people?
We often wonder if the FDA isn’t trying to kill us.
If the FDA approves something that actually works, you can bet that it really, truly works and is high quality. But if they seem to be working on the end of killing humanity, such as dissuading people to use sunscreen or approving of drugs that cause people to develop rapid onset lymphoma, then just ignore the FDA. They obviously sometimes have an agenda that we don’t know that isn’t always honest or good. Sometimes, but not always.
I trust the FDA more than the CDC, which isn’t saying much because the CDC has openly lied for propaganda reasons then retracted their lies as “accidentally placed misinformation” *after* the lies got spread around, but all in all, if the FDA warns about sunscreen absorption, and the effect of not wearing sunscreen is worse, I’m not going to give much weight to the FDA’s warning about the dang sunscreen absorption.
If they develop sunscreen technology so it is better, that’s great. But in the meanwhile, I’m not fretting. No worries.
I find that a good way of checking for possible bias from a single government body is by comparing with the regulations from other countries – as you can see from the percentage comparisons, the US and EU have similar allowed concentrations for sunscreens.
Wow, this is super ignorant. Humira is a biologic that DOES AFFECT the immune system FOR GOOD REASON. I’m not going to simp for Humira or the company that makes it but your take is just bad, bad, bad. Drugs are approved if they improve quality of life with acceptable risks and each person will react differently and have some or none of the ascribed side effects. Humira is NOT prescribed just for “skin disorders and arthritis”. It is prescribed for AUTOIMMUNE DISORDERS (aka disorders of the immune system) like RHEUMATOID arthritis and CROHN’S disease. I will tell you that people who suffer from those disorders will often times (but not always) prefer to suffer some of the “side effects” of a drug like Humira than the lifelong suffering, pain, and misery that those conditions cause. Humira shuts down the immune system to prevent it from killing yourself slowly and painfully while warping your joints or turning your digestive system into a raw bloody tube that feels like you’re being sandpapered from the inside and in severe cases causing 10+ bloody, painful bowel movements a day. Side effects may be worth it, it’s a case-by-case basis and it’s proven more efficacious than other interventions in a significant number of patients. As you may have read on this blog, sometimes different drugs are more or less indicated for different people (given different genetics and pathologies). Given that this is a science-based blog maybe you should not talk about science that you know nothing about? Anyways, not gonna die on this hill, I was just shocked how ignorant this comment was and I had some spare time to express my distaste for it.
Excellent post. Most journalists have no scientific background and are functionally innumerate, so we can’t expect them to report scientific findings with any accuracy.
The European Scientific Committee for Consumer Safety, who advise the European Parliament on legislative measures for cosmetic ingredients, have published numerous toxicological studies about sunscreens (and many other ingredients) over the years – they make informative if highly technical reading, and always have concise, plain-English summaries at the end.
Agreed fully about newer sunscreens; an added bonus is that they’re more stable than avobenzone and oxybenzone. The USA is the only place in the world where they’re not permitted.
When avobenzone was was developed in 1973, it took five years to become legalised in the EEC and 15 years to become legalised in the USA – it’s clear the wheels have always turned very slowly at the FDA.
Michelle you’re an amazing woman with a strong voice thank you for using your skills & knowledge for good! And thank you for all your hard work!!! ??️?
48 rather than 24 subjects? Both sample sizes seem extremely small to me. But I’m an Economist and we usually deem any study below 1,000 subjects as not very credible. I know this differs in science. Still seems very low.
I also hope this gets the FDA to fast-track the approval process for the “newer” organic sunscreen ingredients.
Thanks for writing this up! And for showing the chain sizes. Interesting that ecamsule was also absorbed given its larger size. This makes me wonder what else I put on my face gets absorbed into the deeper layers of the skin and bloodstream. I thought it was nigh impossible for things to do that in the skincare realm, which is why some argue that hyaluronic acid and vitamin c aren’t really as effective. So this is interesting.
As someone with lupus who lives in a very sunny area and has to be very careful with sun protection, I’ll just stick to zinc oxide for now. I have found some that work on my darker skin. But I am eager to try some of these newer filters.
I am particularly keen on getting a sunscreen that I can apply to my body that won’t leave white marks on my black, leather car seats. I’ve only found two that do, but they’re American sunscreens without these newer filters. I heard tinsorb leaves a little white cast still so maybe it’s not the best option – curious if you know if any others that would be good for this purpose while not sacrificing UVA coverage.