I’ve had a lot of people ask me to explain the nitty gritty behind how sunscreen ingredients work. I warned everyone it would be super nerdy and really quite complicated, but a lot of people said it was fine… so here it is: the deep dive into how sunscreen ingredients work!
A warning: this goes into physical chemistry, at upper high school/undergraduate university level. I’m going to try to explain it very simply, so you should be able to follow it even if you don’t remember anything from chemistry in school. But if you’re more interested in finding a sunscreen that works for you, this is probably not the right video – you’ll probably want to look at the other content I’ve done on sunscreen instead: Sun Protection Blog Posts
Here’s the video on YouTube – it has some good animations that I think will really help explain the mechanisms of how these ingredients absorb and emit energy. Keep scrolling for the text version…
Sunscreens and UV
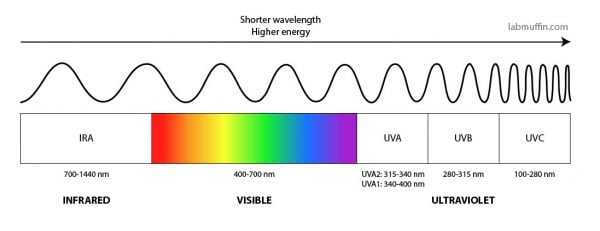
As you all probably know, sunscreens contain active ingredients that stop UV from getting to your skin. Remember that UV is a type of light, with shorter wavelengths and more energy than visible light. There are two main types that we get from the sun, UVA (lower energy, longer wavelength) and UVB (higher energy, shorter wavelength).
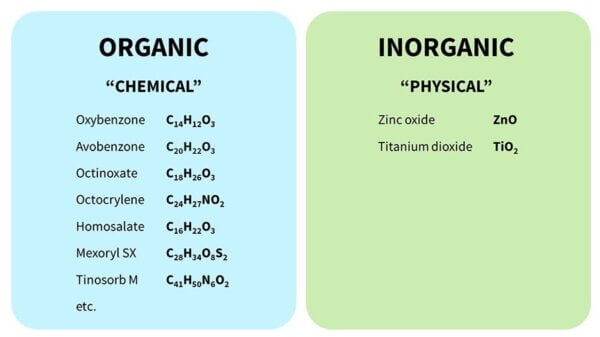
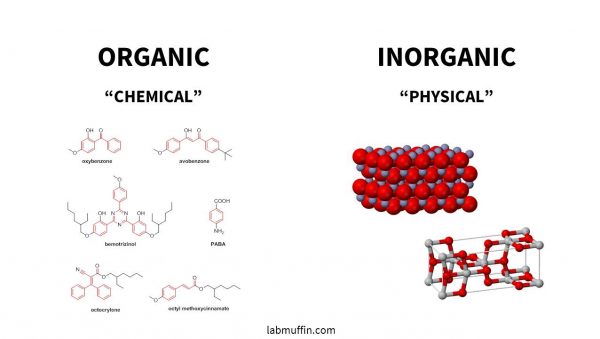
There are two main categories of sunscreen chemicals, organic and inorganic, often called chemical and physical, even though those aren’t great descriptions.
- Organic (“chemical”) sunscreens are the ones with the complicated chemical names, and they have lots of carbon atoms joined together.
- Inorganic (“physical”) sunscreens are zinc oxide and titanium dioxide. These are sometimes called mineral sunscreens.
I talk about which ones you should use in my video about chemical vs physical sunscreen, so if you’re more interested in the applications check that out.
Related post: Chemical vs Physical Sunscreens: The Science (with video)
Organic (“Chemical”) Sunscreens
I’m going to start with how organic sunscreen molecules work. Overall, what they do is absorb UV energy, and convert it to more harmless forms of energy, mostly heat.

The law of conservation of energy says that you can’t create or destroy energy: it can just be converted into other forms. So that’s what these sunscreen molecules do – they’re like little factories that take in UV energy, and turn it into something that’s not going to give you cancer or age your skin massively.
Electrons and energy
The way they do that is with electrons. Chemicals all have electrons inside them, and these electrons (depending on which chemical they’re in) can absorb different types of energy – heat, light, UV – and turn it into other forms of energy.
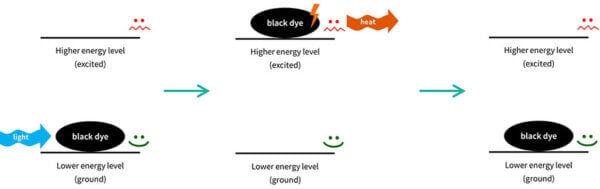
So you can imagine that the sunscreen molecule is sitting on your skin. It has low energy, and it’s very stable and happy to stay there.
When the right type of UV light – the right wavelength – comes along, the electrons in the sunscreen molecule can absorb the energy.
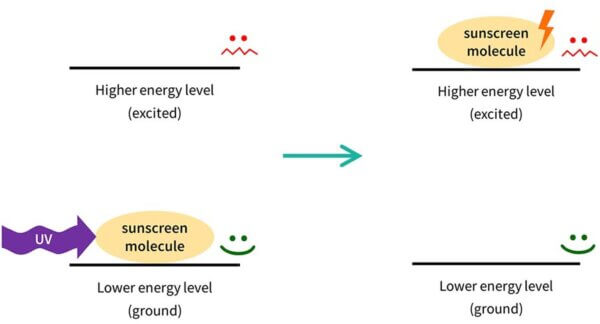
The sunscreen molecule’s electrons now have the energy, so we now we have a very energetic sunscreen molecule – we say that it’s excited (that is actually the technical term).
Things don’t like to have a lot of energy, so this sunscreen molecule isn’t very stable. It wants to get rid of that energy, and go back to where it was before. So the electrons in the sunscreen molecule will emit the same amount of energy back out, usually in a different form. The sunscreen molecule goes back to how it was. This process is called relaxation, which is again, the technical term.
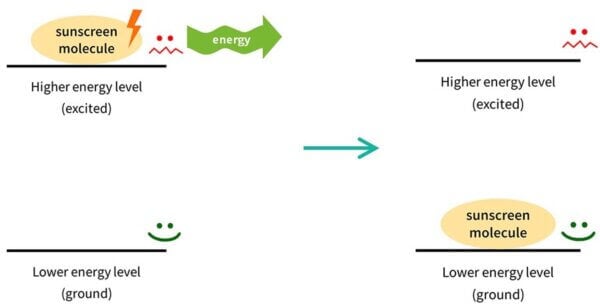
Then it’s back in the lower energy form, so it’s ready to absorb more UV and repeat the process – absorbing UV, emitting other energy – again and again.
This is actually a similar process to why black clothes get hot in the sun: electrons in the black dye molecules absorb visible light from the sun, get excited, then relax and release the energy as heat.
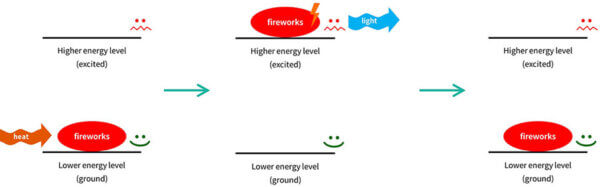
Fireworks also work in a similar way – the electrons absorb heat energy from the explosives, and release it as light. That’s why there are different firework colours – there are different chemicals inside the fireworks that release different colours of light.

What energy is released?
Now you might be wondering – what energy gets released by sunscreen molecules then? It depends on the specific sunscreen molecule, but it’s a combination – it can be:
- Vibrations, which becomes heat when the vibrating molecule knocks into other molecules and the vibrations get passed on (heat is pretty much just microscopic vibrations)
- Lower energy forms of light – remember that you can’t create energy out of nothing, so the molecule might turn part of the UV into lower energy visible light, and the rest of it into heat. Sunscreens can release infrared radiation, which turns into heat in your skin, visible light, or even lower energy UV
- Chemical energy, which means breaking chemical bonds:
- Sometimes this is reversible, so the sunscreen molecule can keep working, so the bond breaks and unbreaks as the UV gets converted like we talked about before (ultimately, the chemical energy will usually turn into heat which dissipates into the rest of your skin).
- But sometimes it’s not reversible, so you can’t get the original molecule back, and the sunscreen slowly decomposes and stops working over time. This is what happens when a sunscreen is photounstable. When formulators make a sunscreen ingredient more photostable, they’re basically doing things to the formula to try to convince the sunscreen molecule to make the other types of energy, instead of breaking the irreversible bonds.
How much heat are we talking about?
With all this talk of heat, you might be wondering: does your skin heat up?
The answer is maybe a tiny bit – but the amount of heat is tiny compared to the rest of the heat you’re getting from other things. If you’re wearing sunscreen and you can feel your skin get hot when you’re in the sun, it’s probably the infrared and visible light that you’re feeling turn into heat, not the UV. Sunlight is only about 3-7% UV, and about 44% visible light and 53% infrared.
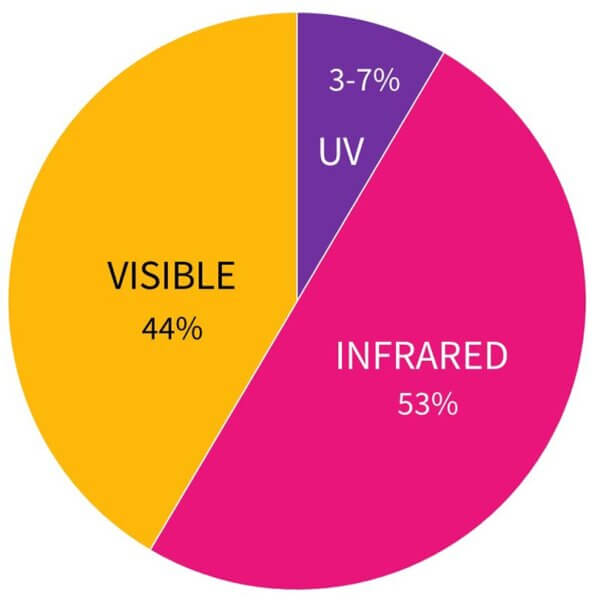
Your skin might also be irritated by the sunscreen, which makes it go hot. But it’s almost definitely not the UV you’re feeling – if you convert all of the UV hitting your skin into heat, it’ll go up by less than one degree, and this is largely localised to the very surface layers of your skin (whereas visible light and infrared get converted into heat much deeper in your skin). There’s probably a bigger temperature difference between your upper and lower arms right now.
Which wavelengths get absorbed?
So organic sunscreen molecules absorb UV – the energy they absorb matches the energy gap between the lower and higher energy levels.
But why do some sunscreen molecules have energy gaps that can absorb UVA, some that can absorb UVB and some that can absorb both?
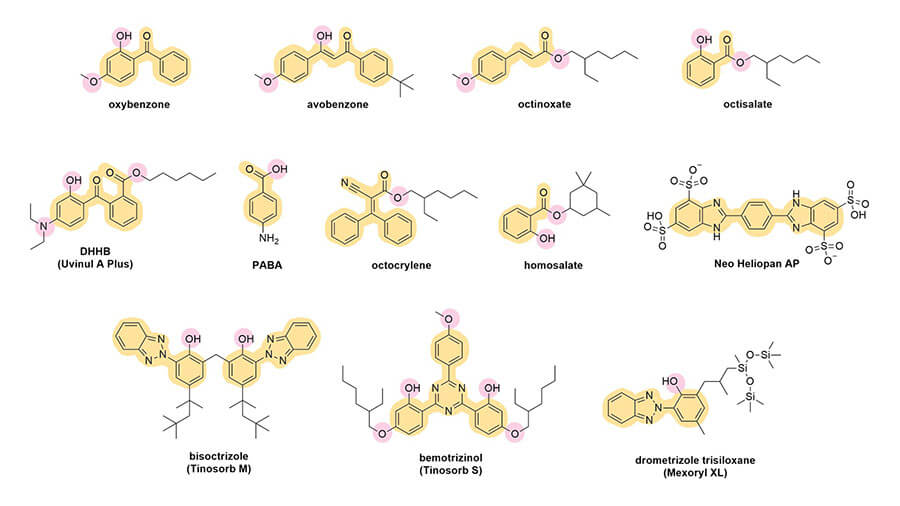
Let’s look at their structures:
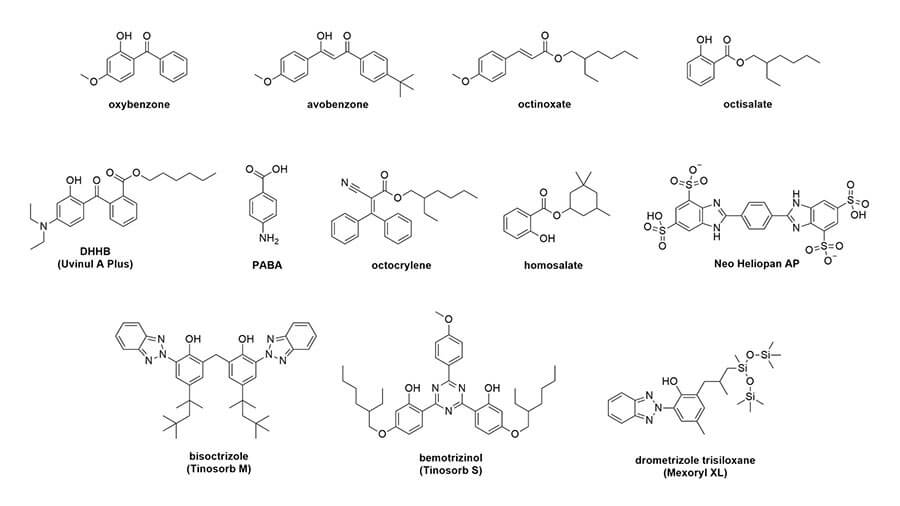
Conjugation, delocalisation and wavelengths
The first thing you’ll probably notice is that they all have a lot of rings – mostly hexagons and pentagons. They also have this pattern of alternating double bond, single bond, double bond. This is sometimes in the rings, sometimes not. This is called conjugation. Here’s what it looks like in octinoxate:
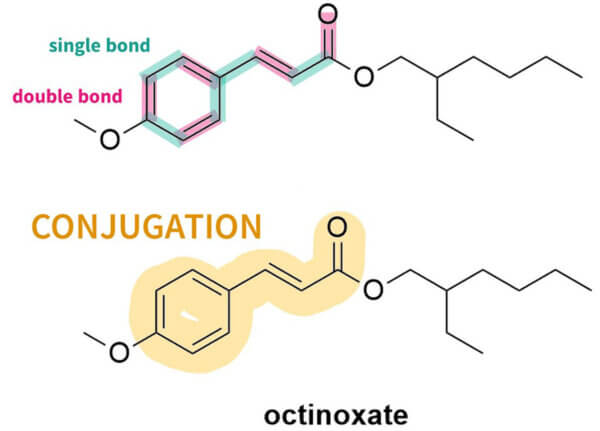
And you can see this in the rest of the sunscreen structures too:
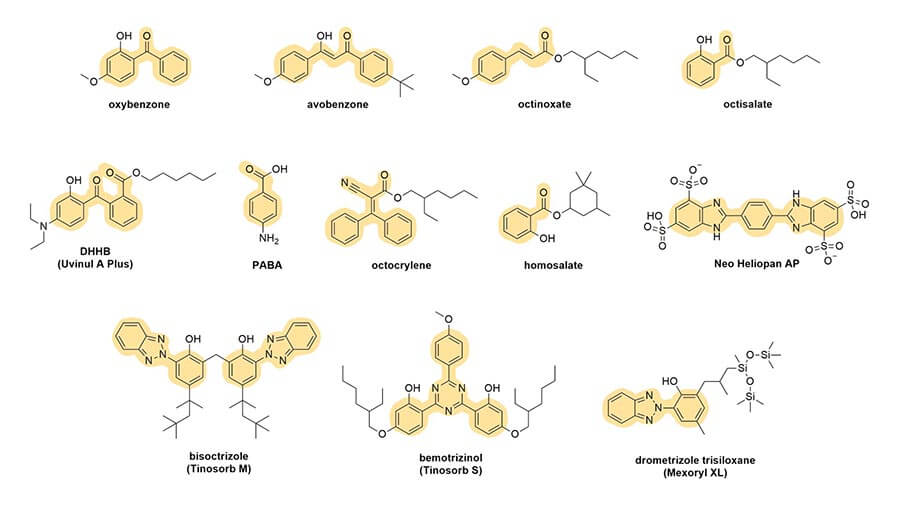
Conjugation gives the electrons in the molecule a special property – they can run around a bit, which is called delocalisation.
The bigger the conjugated pattern, the more the electrons can run around and be delocalised, and the smaller the amount of energy it can absorb. In short, greater delocalisation means a smaller energy gap that absorbs longer wavelengths.
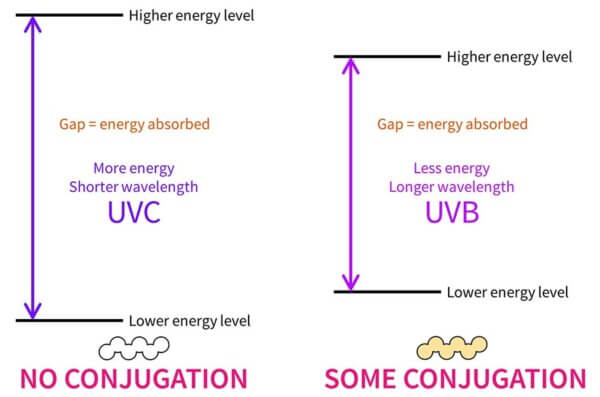
By default, if you have a molecule with no conjugation it’ll usually only be able to absorb high energy UVC. In other words, the energy gap between the lower and higher energy levels matches the UVC range. This is completely useless for us, since UVC gets absorbed by the atmosphere before it reaches us on earth.
If it’s a smaller molecule with conjugation, this gap gets smaller and it’ll absorb lower energy UVB.
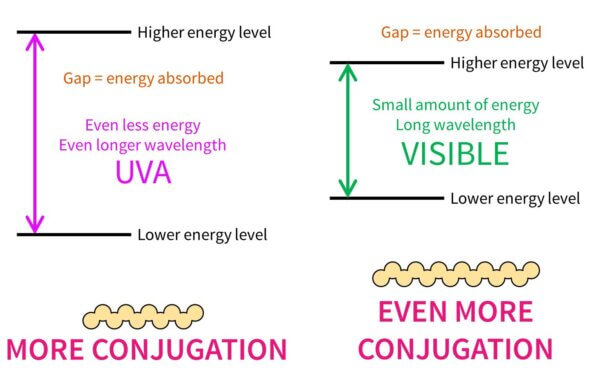
If the conjugated pattern gets even larger, the energy gap will get even smaller and it’ll absorb UVA – you’ll notice that UVA filters tend to be larger than UVB filters. Rings also increase the delocalisation, which is why you see all these rings around. Visible light has less energy than UVA, so it makes sense that coloured dyes, like hair dyes, are even bigger.
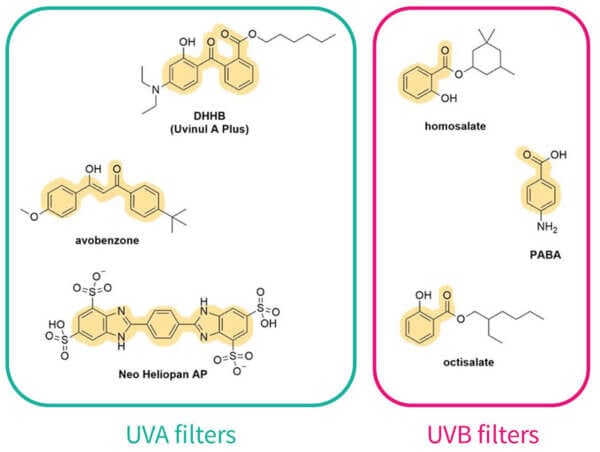
Other structural features
Other things on the sunscreen molecule can also change the amount of delocalisation. Oxygen and nitrogen atoms can add extra electrons, so this extends how far the electrons can run around and the delocalisation gets larger.
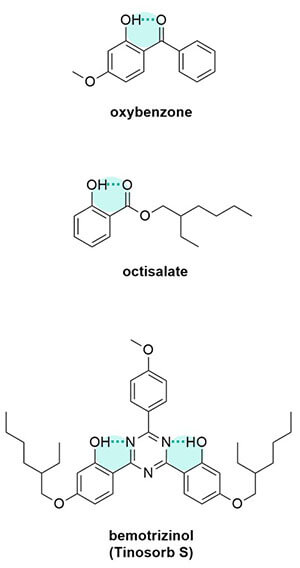
If atoms on the molecule can interact and form more rings, it gives it more delocalisation too.
Let’s look at avobenzone as an example – avobenzone can be in two forms, and they can turn into each other.
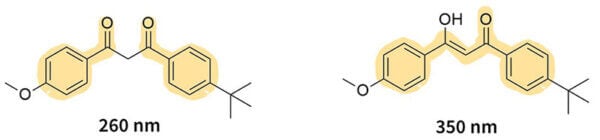
On the version on the left, there are two single bonds next to each other in the middle, which breaks the conjugation. But in the version on the right, the conjugation continues through almost the whole molecule. So the first version is less delocalised and absorbs more energy (a shorter wavelength of 260 nm), and the second version absorbs less energy (a longer wavelength of 350 nm).
Influence of formula
This concept – that the wavelength a molecule absorbs depends on its delocalisation – also explains some of the things about the importance of formula that people talk about.
pH
For example, acidic filters at high pH will get a negative charge, which means there are more electrons, more delocalisation and it’ll absorb a longer wavelength. Basic filters – these usually have a nitrogen in their structure – at low pH will get a positive charge, which cuts the delocalisation short and it’ll absorb a shorter wavelength than if it was at a higher pH.
Solvents
Solvents can also interact with either the higher or lower energy level to make it lower in energy. If the solvent interacts with the lower energy ground state, then the gap gets bigger and it absorbs a shorter wavelength. If it interacts with the excited state and lowers than then the energy gap gets smaller and it absorbs a longer wavelength. Octinoxate’s main UV absorbance can actually change by about 20 nm just by changing the solvent.
UV absorbance curves
So let’s ungracefully switch for a minute to these graphs that you might’ve seen around before:
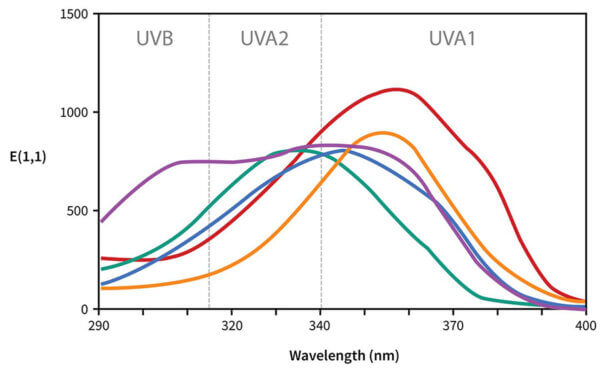
Each line shows the UV absorbance for a specific sunscreen ingredient. I’m going to get rid of most of these and just leave avobenzone here:
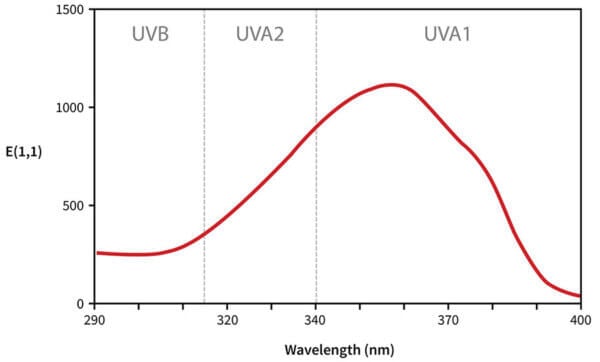
The way we read this graph:
- The horizontal axis is wavelength. As we go from left to right, the wavelength gets longer, so it goes from high energy UVB to lower energy UVA.
- The vertical axis is absorbance, so the higher the line is, the more of that wavelength it’s absorbing.
So avobenzone absorbs some UVB, absorbs more UVA, absorbs the most at 357 nm, then absorbs less as we get closer to the end of the UVA region. That’s why we call it a UVA filter – you can see it does absorb UVB, but it mostly absorbs UVA.
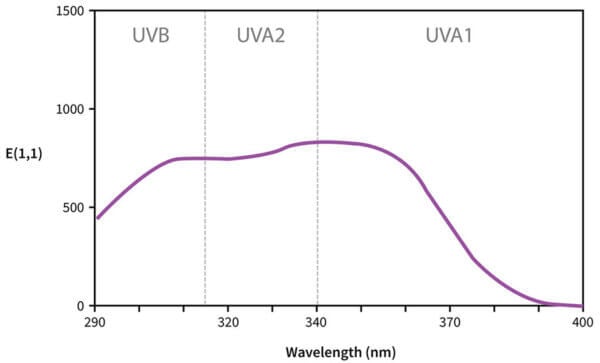
A broad spectrum sunscreen ingredient would have a wider curve, so it absorbs the wavelengths more evenly. For example, Tinosorb S has a chunkier curve, so it’s broader spectrum, it absorbs both UVA and UVB efficiently.
Why are they curves?
Now, you might be wondering – if the molecule is absorbing energy that matches the energy gap, and we only have one molecule, shouldn’t avobenzone only absorb the one wavelength that matches the energy gap? Why is it absorbing some at 357 nm, and some at 370, and some at 300? Why don’t we have a UV absorbance dot, with one wavelength absorbed, instead of a curve?
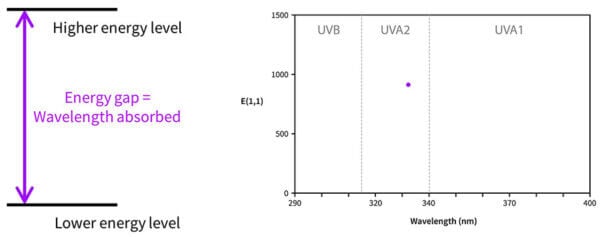
It’s because in real life, molecules move around, and in a drop sunscreen there are billions and billions of sunscreen molecules that are orientated slightly differently. All of these will have slightly different delocalisations and absorb slightly different wavelengths.
So the energy gap diagram really looks more like the diagram on the right, with lots of energy gaps possible.
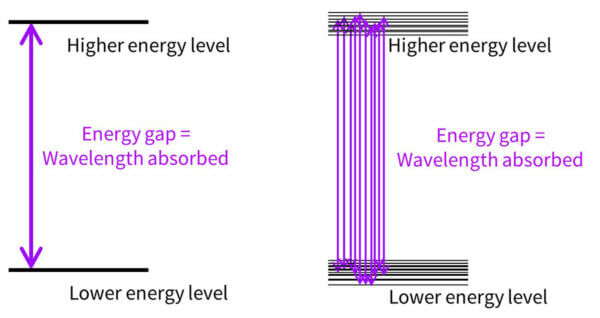
More of the 357 nm transition happens, there are a bit less of the 350 nm transition, less of the 340 nm transition and so on – building a curve. And this happens with every sunscreen molecule, which is why every absorbance curve is a curve, and not just a dot – all of these molecules absorb lots of different wavelengths in different amounts.
Now, some of these curves are more complicated. Let’s have a look at Tinosorb S again – it has a double hump.

This is actually because there are two overlapping curves here. Tinosorb S and many of the other newer sunscreens (and some of the older ones) are a bit more complex – their delocalisation patterns look a bit more like a 2D shape instead of a more 1D line like avobenzone. So there’s more than one main electronic transition that Tinosorb S undergoes: one is in the UVA region, and one is in the UVB region, and both of them are again a bit fuzzy so you get a curve instead of just two dots.

Now if we compare the absorbance curves of different sunscreen filters, you can see that some are higher and some are lower. The higher the line is, the more it absorbs of that particular wavelength, so a higher line in the UVB region means higher SPF, higher in the UVA region means higher UVA protection.
These lines all compare the same mass concentration of sunscreen (1% in ethanol), so this very roughly reflects what happens with the actives of an actual sunscreen product. But a lot of factors are different in actual sunscreens – the filters will have different properties floating around in ethanol, compared to after application on skin and evaporation of the solvent, and formation of a thin film. And remember there are a lot of things like pH and solvent that can change the shape of the curves, so the curve might shift to the left or right, or up or down.
It gets even more complicated if you have a sunscreen containing more than one filter – if you put a UVB and a UVA filter together, you can get a broad spectrum sunscreen. The curve for the final sunscreen will look very different, and it can be very difficult to predict – you can roughly add them, but the two filters can interact synergistically so the curve ends up higher than expected, they can interact with each other and change the delocalisation so they might absorb a different wavelength. There are lots of possibilities that you can’t easily predict.
Concentration is also important – these curves are for 1% in a solution, not whatever concentrations you have in an actual sunscreen – if you have a higher percentage the curve will go up, because you have more sunscreen molecules on your skin, so the mixture can absorb more UV.
(There are other considerations in a real sunscreen that I haven’t talked about here, like SPF boosters and film formers – I discuss those a bit in this post.)
Another thing to notice is that it doesn’t matter how many sunscreen filters you use to build the final curve, what matters is the final curve. I’ve seen skincare science influencers say that you need more filters for redundancy, but that’s not how UV absorbance works. It’s like saying that a 2 metre ladder made of steel, wood and aluminium will get you higher than a 2 metre ladder made of just steel – the materials don’t make a difference to the final height.
Inorganic (“Physical”) Sunscreens
Now onto the inorganic sunscreens, zinc oxide and titanium dioxide.
Back in the day the cosmetics industry thought that they worked by just scattering UV. It was even written into the 1978 FDA monograph, but then it was taken out in the 1999 version – this is probably why the myth is so widespread.

But scientists have known that they work by both absorbing and scattering UV (mostly absorbing UV) for a long time, and peer-reviewed papers keep pointing out that the scattering and reflecting mechanism is a misconception, but like a lot of myths it just refuses to die.
UV absorbance
The way that zinc oxide and titanium dioxide mostly work is a lot like organic sunscreens. They have electrons that can absorb UV and go into a higher excited state. The electrons can then relax down while releasing the energy as more harmless forms – heat, IR, visible light, UV.
But these ingredients are inorganic, so they don’t have carbon atoms and don’t have the same ability to contain conjugated systems Instead, they have what’s called a band gap structure.
If you look at the structure of zinc oxide, there are a lot of atoms, which have lots of electrons, and they all interact with each other. This means there are lots of slightly different energy levels packed very closely together.
So instead of a lower energy level you have a lower energy band made up of lots of levels, and a higher energy band made up of lots of levels:
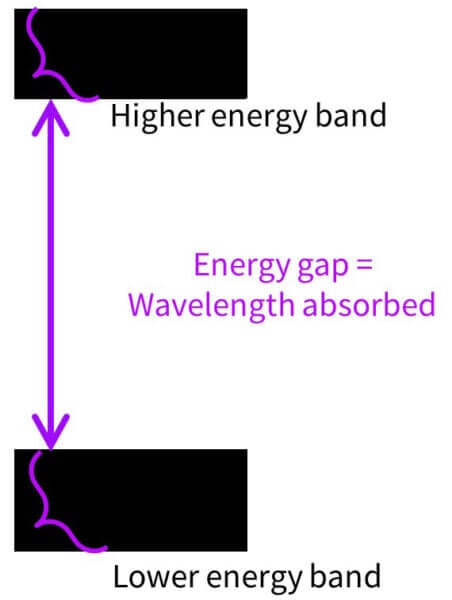
The smallest gap between them, from the top of the bottom band to the bottom of the top band, is the minimum energy or longest wavelength that can be absorbed. They can absorb pretty much every UV wavelength with more energy than this, because there are so many energy levels. So that’s why the absorbance curves for zinc oxide and titanium dioxide look like they suddenly jump off a cliff – they stop absorbing at the shortest gap.
UV scattering
Now the electrons and energy levels explains about 95% of how they work – the rest of it is scattering.
Scattering is when the UV comes in and the particle changes the direction the UV is going in.
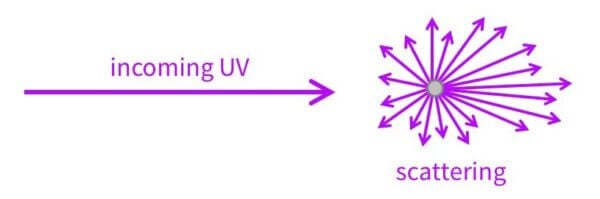
Scattered UV doesn’t always bounce backwards, it actually usually goes forwards. So a lot of the time UV light actually gets scattered towards your skin… but that still ends up decreasing UV exposure, because a lot of it hits another sunscreen particle and gets absorbed or scattered some more.
Complications with particle size
Inorganic sunscreens are a bit complicated, because you can get different particle sizes. In sunscreens the particles are usually really small – less than 200 nm in diameter.
That’s mostly because scattering is proportional to diameter. Larger particles scatter more and absorb less, and it isn’t just UV light that gets scattered – it’s also visible light. So the larger the particle, the more scattering you get, and the more of a white cast you see.
There’s also the fact that only the atoms on the surface of the particles absorb UV. If you have a larger particle and break it down into smaller pieces, more surface area gets exposed, so more UV can be absorbed.
Longer wavelengths also get scattered more and short wavelengths get absorbed more, so even though these ingredients are mostly absorbing UV, they can still give you a white cast. Zinc oxide absorbs both UVB and UVA, while titanium dioxide mostly absorbs UVB and mostly scatters UVA. But it does depend on the particle size used in a particular sunscreen.
I’m going to leave the explanation here – hopefully it was understandable!
References
Chem Guide has fantastic explanations of chemistry concepts – here’s their explanation of UV absorbance and UV curves. Most first year university chemistry textbooks have information on UV spectroscopy.
Lowe NJ, Shaath NA & Pathuk MA (eds), Sunscreens: Development: Evaluation, and Regulatory Aspects, Marcel Dekker, 1997.
Wang SQ, Lim HW (eds), Principles and Practice of Photoprotection, Cham: Springer, 2016.
Cole C et al., Metal oxide sunscreens protect skin by absorption, not by reflection or scattering, Photodermatol Photoimmunol Photomed. 2016, 32, 5-10. DOI: 10.1111/phpp.12214






Yes, understandable, but only because you did explain it very well and in easy terms – it surely is complicated. Thank you for breaking it down!
Michelle, thank you for the thorough explanation!
I would love to see a post from you breaking down how laser hair removal affects skin.
Thanks for the explanation. What I am wondering since I know that physical sunscreens reflect the UV-light is whether you can add make-up, like blush, bronzer, powder, over it. I am affraid that the sunscreen will no longer reflect the UV-light and still burn your skin.
Can you pleas say something about this Michelle? Thanks a lot!
Regards, Jouke
Physical sunscreens don’t reflect UV, they work primarily by absorption.
This was interesting, so the main difference between the “physical” and “chemical” sunscreens is just that one is molecular and one is crystalline? Out of interest (feel free not to answer if you don’t have time), you mentioned that Zinc Oxide and Titanium Dioxide are the physical sunscreens. Are there other insulators that might work or do these have band gaps that are particularly useful for UV absorption? Is the scattering more dominant if you were to use metal oxides or is it still small?
Sorry, this sent me down a bit of a rabbit hole the more I thought about it.