These days we’re all aware that suntans give you both cancer and wrinkles, so fake tan is the colour du jour. This miracle-in-a-can stains the skin brown through an interesting chemical reaction. The outer layer of your skin, made up of dead skin cells, is permanently coloured. The tan wears away as the skin cells come off. Here’s how it works, and whether it’s safe!
The Chemistry of Fake Tan
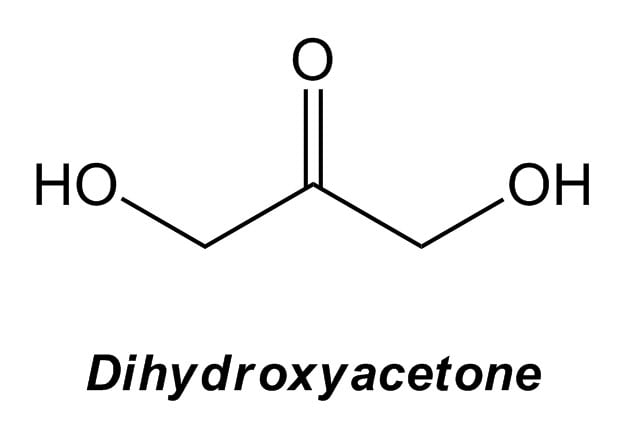
Fake tan products you find in stores contain 2-5% dihydroxyacetone, which looks like this:
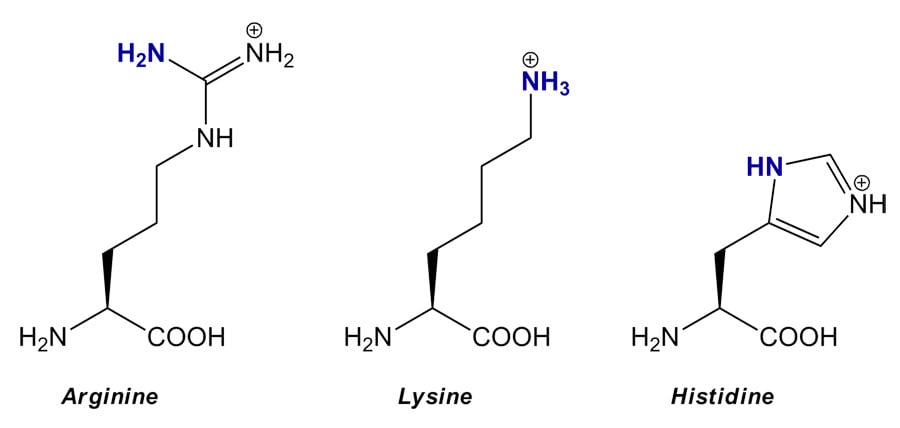
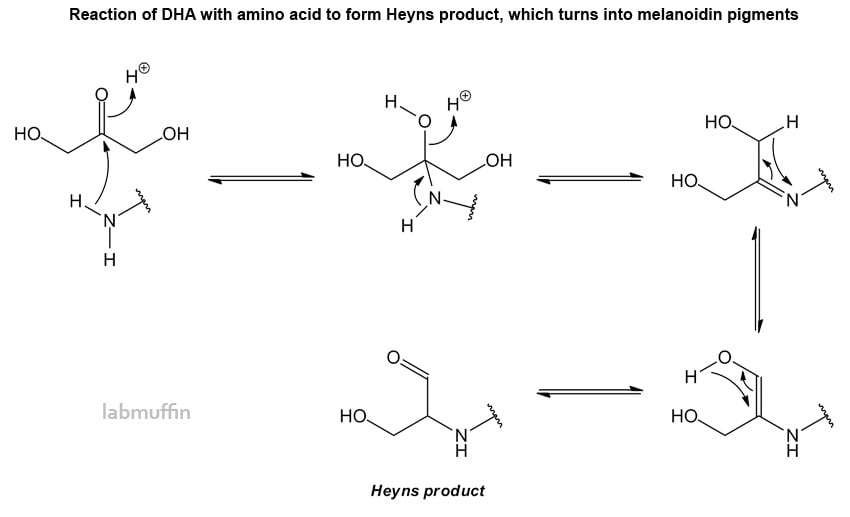
It starts off colourless, but it reacts with amino acids (particularly arginine, lysine and histidine) in the skin to form a variety of brown compounds called melanoidins.
It’s actually the same chemical reaction as the one responsible for making food like bread and meat turn brown and delicious when cooked. It’s called the Maillard reaction. For the really intense chem nerds, it proceeds like this with DHA (I got a bit lazy, sorry for the shortcuts):
This forms covalent bonds, which means the skin is permanently stained – water, soap and moisturiser won’t wash it off. The skin starts devloping the tanned colour after 2-3 hours, and the reaction continues for the next 1-3 days. The reaction occurs best at moderately low acidic pHs (3-6), so fake tans tend to come in this skin-friendly range. The extent of the reaction is also influenced by the amount of water around.
Application and Aftercare
DHA only penetrates the very top layer of skin (the stratum corneum), which you may know is dead skin cells. This is why fake tans can’t last longer than about a week – that’s about how long it takes for the stained skin to wear off (that’s why if you look up fake tans that claim to last longer than a week or two, you’ll find tons of grumpy reviews – the skin sheds at a similar rate no matter what product you use!).
This explains all the advice given for making your tan look good and wear off evenly:
- Apply with a mitt – If you apply with your hands, you’ll stain the thick skin on them, leaving them grubby-looking.
- Exfoliate before you tan – Since the DHA has to migrate into the skin to stain, having uneven skin means it’ll sink in unevenly and give you a splotchy tan.
- Shave and wax before you tan, avoid it afterwards – Shaving and waxing will both exfoliate your skin, removing the stained layer. Shaving scrapes off an uneven layer of skin, while waxing pulls it off.
- Don’t let it get wet for a few hours – Any uneven moisture will affect how well the reaction proceeds, making it streaky.
- Exfoliate lightly a few days afterwards – if the skin builds up too thickly, it’ll come off in unsightly clumps.
- Moisturise – this helps the skin wear off evenly.
Colour
These days fake tans are less orangey-hued than the fake tans of the past, due to smarter formulations and purer DHA sources. For example, Rimmel Sun Shimmer in Dark (aka the first and only fake tan I’ve tried) came out as a slightly green-hued foam. The green counteracts the unsightly redness that pulls the tan orange, since they’re opposites on the colour wheel. This leaves a natural golden-brown tan colour instead. In this photo my leg is on Tan Day 2 but my arm is bare:
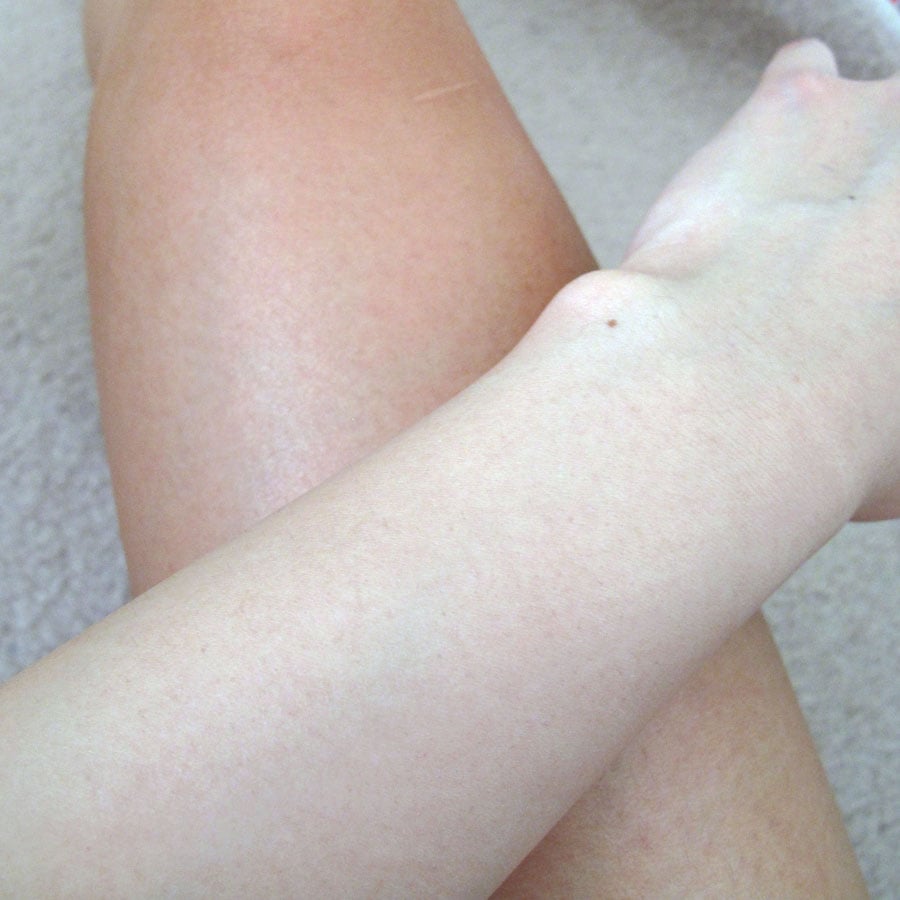

Is it safe?
- There’s been some studies indicating that it could potentially be carcinogenic if inhaled, but in unrealistically large amounts.
- If you go insane using self-tanner daily, after about a month you could end up with severe irritation according to this study… so don’t do that.
- The biggest risk is probably the fact that people think a fake tan will protect from the sun – it doesn’t! It gives about SPF 2 or 3 – about as much as if you politely asked the sun not to burn you. You’ll still need lots of sunscreen!
- This study found that 180% more free radicals were formed during sun exposure after 20% DHA was applied to skin. Free radicals are the species which cause photoageing (sun-induced wrinkles and other signs of ageing). Some people have freaked out about this, but 180% is only 3 times the usual amount, and 20% DHA is a lot more than usual. It’s also very easy to prevent – either stay out of the sun after using self-tanner, or use sunscreen (which you should be doing anyway)!
References
BC Nguyen, IE Kochevar, Influence of Hydration on Dihydroxyacetone-Induced Pigmentation of Stratum Corneum, Journal of Investigative Dermatology 2003, 120, 655-661 (full text)
SP Foltis, Formulation and evaluation of self-tanners, Cosm & Toil 2012, 127, 442-445 (full text)
K Jung, M Seifert, T Herrling, J Fuchs, UV-generated free radicals (FR) in skin: their prevention by sunscreens and their induction by self-tanning agents, Spectrochim Acta A Mol Biomol Spectrosc 2008, 69, 1423-1428 (abstract/full text)
T Kimura, Contact dermatitis caused by sunless tanning treatment with dihydroxyacetone in hairless descendants of Mexican hairless dogs, Environ Toxicol 2009, 24, 506-512 (abstract)






As a pale girl and an avid fake tan user I found this post extremely interesting! I have never really put much thought into how a tanner stains the skin but knowing that it is a chemical reaction with the skin makes a lot of sense – and also makes me feel a bit squeamish I must admit! But that Rimmel tanner is definitely one my faves at the moment!
Thanks for this post! x
Chantalle | cece & grace
Ahhh all that electron pushing. I definitely do not miss those days in organic chem! This reminds me, I should tan my white legs!
Michelle, could you briefly explain the science behind why DHA generates more free radicals (I couldn’t understand the terminology in the study you linked and you’re so much better at explaining)? Will sunscreen (with zinc) completely neutralize the risk?
I also wanted to add that I LOVE your blog! It’s so refreshing to be able to read up on and understand the actual science behind how/why beauty products work.
Essentially it creates chemicals in your skin that are unstable in UV light. Sunscreen will almost completely cancel this effect out! 🙂
Thank you so much!
Michelle, it’s good to know the science behind tanning lotions. And you made the explanation so easy to understand. Thank you for the information.
Wow, this was a really interesting read and very informative! Sunless tanning is an excellent alternative to tanning in the sun or in a tanning bed. It certainly is less risky and can even produce better results at a much faster rate. It was really cool learning about how it works from a scientific standpoint! Awesome post!
Simple self explanatory article. Thanks Michelle
What about the free radicals that are created without UV light? How do we fight those?
Wow, what a comprehensive post, you made it easy to understand. Love it!
Hi Lab Muffin,
I’m wondering what your thoughts are regarding self tanners and whether they can cause skin to age more rapidly. I see that you explain why going in the sun is a bad idea, but can a self tanner actually age skin even if you stay away from sun exposure? Thanks in advance!
I don’t think so – DHA reacts with the upper layers that are quite dead and about to shed, which is why the tan fades so quickly. I doubt there’s any impact lower in the skin, and I don’t think there’s any actual evidence for that happening other than vague speculation.