One thing that a lot of cosmetic companies have recently been incorporating into their marketing talk is the concept of pH and pH balance. Yay science! But what does it all mean? Here’s a quick rundown on the science of pH, acids and bases, and what that means for your skincare products.
What is pH?
pH is a measure of how acidic something is. It’s thought to stand for “power of hydrogen” (although scientists disagree about the exact origin of the abbreviation). Acidity is essentially the ability of something to donate hydrogen ions, H+. Specifically, for pH, this is the ability to donate H+ ions in an aqueous (water-based) solution.
If something is acidic, it will:
- be able to donate H+ ions
- taste sour
- have a pH less than 7
If something is basic (also sometimes called alkali or alkaline), it will:
- not be able to donate H+ ions easily, and in fact they’ll usually absorb H+ ions or donate hydroxide ions (OH–)
- taste bitter
- feel slippery
- have a pH greater than 7
Most things will have a pH of between 0 and 14, although concentrated acids and bases can have a pH value below 0 or above 14. Substances at either extreme will be corrosive – they’ll give you a chemical burn, and burn holes in other things, like your clothes!
Related post: It’s The Lab Muffin Guide to Basic Skincare!
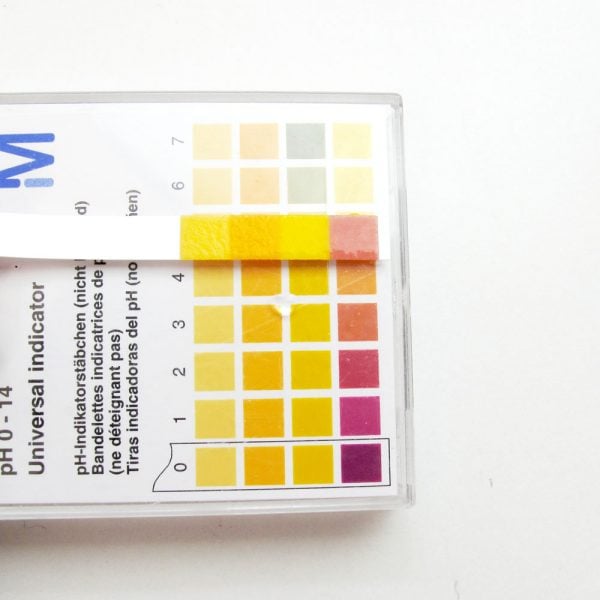
The approximate pH values of some common substances are:
- Battery acid = 0-1
- Stomach acid = 1-2
- Lemon juice = 2-3
- Vinegar = 2-3
- Fizzy drinks = 3
- Orange juice = 3.5
- Shampoo = 5.5
- Normal skin = 4-6
- Pure water = 7
- Blood = 7.4
- Seawater = 7.5-8.4
- Toothpaste = 8
- Baking soda slurry = 8-9
- Standard soap = 9-10
- Milk of magnesia = 10.5
- Ammonia = 10-11
- Oven cleaner = 13-14
- Lye = 13-14
The pH scale is a log scale
The scientific definition of pH is:
pH = -log10[H+]
What these symbols mean:
- [H+] stands for the concentration of H+ ions in the substance in moles per litre (the standard unit for concentration used in chemistry)
- log10 means the base 10 logarithm
Mathematically, this means that the lower the pH the more H+ ions there are, and each pH unit represents a 10-fold difference in concentration.
For example, something that is pH 1 doesn’t just have double the concentration of H+ ions as something at pH 2 – it has 10 times the concentration!
If you want to dilute a very acidic substance from pH 2 to pH 5, you’ll have to dilute it 1000-fold (i.e. you’ll have to add 999 mL of water to 1 mL).
pH balanced skincare
So what does this mean for pH-balanced skincare?
There are two main situations where the pH of a product can make a big difference in your skincare: when the product is a cleanser, and when the product has to be at a certain pH to work.
pH and Cleansers
Our skin is actually quite amazing. On the surface, there’s a layer called the stratum corneum, which protects us from injury, infection and dehydration. It’s made up of keratin, dead cells and secretions, such as sebum. Its pH is usually slightly acidic, but with some conditions (e.g. eczema, diabetes) the pH tends to be higher.
For people with sensitive skin, including infants and the elderly, large changes in pH can lead to disruption of the stratum corneum. This can translate to dryness and irritation. For the rest of us, our skin is still pretty resilient and can readjust its pH after exposure with most products.
The only issue is when you have a cleanser, which contains surfactants. Since these disrupt your skin barrier, it makes it more susceptible to pH disturbances, so even normal skin can’t readjust its pH back quickly. Repeated disturbances can make your skin less resilient and more prone to dryness, irritation and acne.
Because of this, alkaline soaps and cleansers above pH 7 should be avoided by most people, since these products have more lipid-stripping power and can change the pH of your skin for prolonged periods (see e.g. face washing science).
Luckily most skincare companies realise this now, and most skincare products are pH balanced, even if they don’t say they are. I personally don’t think should you pay extra for skincare labelled with “pH balanced”. It’s much more reliable to look at the actual pH of the product (ask the brand if they’re willing to tell you, or test it yourself with pH strips).
For more information on how to choose a cleanser (checking for pH, but also ingredients and format and all the other nitty gritty), you can check out The Lab Muffin Guide to Basic Skincare.
pH and active ingredients
For some active ingredients, the pH will be important to how well it’ll absorb into your skin. This is particularly an issue if it’s a small molecule with an acidic or basic functional group. This topic is quite technical, so I’ve made a few other posts and videos on the topic – check them out below!
- Why does pH matter for AHAs and acids in skincare? (video)
- Free Acid Calculator for Exfoliants at Specific pH Levels
- Fact-check: Why does pH matter for AHAs and BHAs?
[lastupdated]




If you had written this post 5 years earlier, maybe I wouldn’t have struggled so much with Chemistry 😛 My teacher couldn’t explain anything and I ended up hating the subject! But reading this, it makes sooo much sense. Thanks 😀
Haha thanks! 😀 I do actually enjoy teaching chemistry a lot… I am the biggest nerd! (But I have exciting nails, which makes it all ok, right?)
Oh, and of course, if there are any topics you’re interested in having me cover, let me know! 🙂
Haha, this is the only science lesson I’ve willingly sat through. Never thought about what pH was, now I know! If only all science was taught this way 😉
Mission accomplished! 😉
This is great and all makes sense! I love your scientific snippets 🙂
I nominated you for the “I love your Blog” award…check it out here: http://hookedbyhollysagemini.blogspot.com/2012/01/i-love-your-blog-award.html
Oh wow thank you! 🙂
omg i LOVE this. i am such a HUGE bio nerd (and polish junkie) i love reading all your science stuff! soooo excited to follow your blog! 🙂