Sodium hydroxide is in a ton of beauty products. But as one of my favourite unreliable sources says (the “favourite” is sarcastic by the way, just to clarify):
Sodium Hydroxide is, however, a known irritant…
The National Institute for Occupation Safety and Health … recommends that consumers prevent skin and eye contact
The CDC reports that “Skin contact with sodium hydroxide can cause severe burns with deep ulcerations. Pain and irritation are evident within 3 minutes, but contact with dilute solutions may not cause symptoms for several hours.
Solutions as weak as 0.12% have shown to destruct healthy skin cells within one hour.
– Truth in Aging
It sounds pretty nasty, and it’s nasty even at a super low concentration of 0.12%!
But then why is it in everything? Let’s talk basic (haha) chemistry…
What Does Sodium Hydroxide Do?
Sodium hydroxide, with the formula NaOH, is usually used in products as a pH adjuster. It’s also used in soap-making to turn fats and oils into soap. You can find sodium hydroxide (also called caustic soda or lye) in its pure form in the cleaning aisle, since it’s also really handy for clearing up clogged drains.

But here’s something interesting…
Sodium Hydroxide Isn’t Really in Most Products
To really understand why we don’t need to freak out about sodium hydroxide, we need to understand what happens when we put sodium hydroxide into a product.

Sodium hydroxide is a soluble inorganic compound and a strong base, and this makes it really unusual because, unlike most other ingredients, the amount you put into a product isn’t the amount you end up with in the product at the end.
Sodium Hydroxide Chemistry
When sodium hydroxide dissolves in water, it breaks up (dissociates) into sodium ions (Na+) and hydroxide ions (OH–):
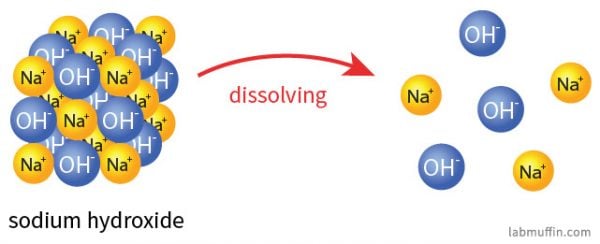
The sodium ions are pretty benign. Table salt is sodium chloride (NaCl), and when that dissolves in water (or in your food), it splits up into sodium ions (Na+) and chloride ions (Cl–). The sodium ions from sodium hydroxide and the sodium ions from salt are indistinguishable after the substances have broken up:
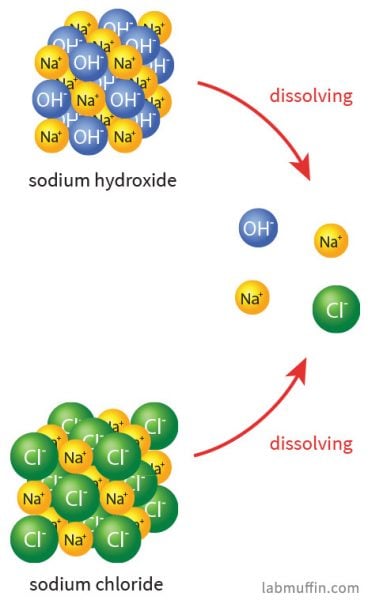
There’s tons of sodium ions everywhere: there’s almost 1 gram per litre of sweat, and seawater is 1.08% sodium ions by mass. We have lots of sodium ions in our bodies, and it’s essential for the way our body functions.
If you don’t have a high enough concentration of sodium ions in your blood, you end up with hyponatremia, which can kill you (the name comes from natrium, the Latin name for sodium). This is the reason that water in large doses is toxic.
It’s really the hydroxide ions that are the cause of the trouble.
Hydroxide Ions
We don’t talk much about hydroxide ions in skincare, and we really should!
We talk about hydrogen ions (H+) a lot, since hydrogen ions are the reason for acidity. pH is a measure of the concentration of hydrogen ions in a water-based substance. If you don’t know much about pH yet, check out this guide to pH that I wrote. (Don’t worry, this post will still be here when you get back!)
The key things about pH you’ll need to know to understand what comes next:
- pH 7 means neutral. If it’s lower than 7 it’s acidic, and if it’s higher than 7 it’s basic.
- Lower pH means a higher concentration of H+ is present.
- Acidic substances donate H+ ions in water.
- Alkaline substances absorb (accept) H+ ions in water.
- The formula for calculating pH is pH = -log10[H+].
(Side note: Hydrogen ions are more correctly referred to as hydronium ions, but for the sake of simplicity I’m sticking with hydrogen ions.)
Here’s the thing about hydroxide ions – they react with hydrogen ions to form water:
H+ + OH– → H2O(l)
This is how they work as pH adjusters – they neutralise the hydrogen ions and turn them into water, raising the pH of the product. Most of the added hydroxide ions will turn into water, and sodium ions will float off.
Water can also break apart to form hydrogen ions and hydroxide ions:
H2O(l) → H+ + OH–
This is why pH 7 is neutral, even though you can get less hydrogen ions if you have a higher pH. At pH 7, you have the same amount of hydrogen ions and hydroxide ions (at 25 °C). At lower pH you have an excess of H+, and at higher pH you have an excess of OH–.
Because hydrogen ions and hydroxide ions are linked to water in this way, you can actually work out the concentration of hydroxide ions in a product if you know the pH. The formula is a little bit complicated:
Concentration of hydroxide ions ([OH-]) = 10-(14-pH) mol L-1
Basically it’s just the opposite of pH. pH 14 has the same concentration of hydroxide ions as pH 0 has of hydrogen ions, and so on.
What does this mean for sodium hydroxide in skincare products?
Here’s the nitty gritty.
The only reason sodium hydroxide can be harmful in products is because of the hydroxide ions, and you know exactly how dangerous the hydroxide ions are if you know the pH of the product.
If the product is at pH 7, it has the same concentration of hydroxide ions as water (i.e. not very scary). You don’t need to worry about sodium hydroxide.
If the product is at a pH below 7, it has less hydroxide ions than plain water (still not very scary… but if you go too low then you need to start worrying about hydrogen ions). You don’t need to worry about sodium hydroxide at all.
If the product is at a pH above 7, then it will have more hydroxide ions than water. In this case, look at the pH – the higher the pH, the more dangerous the hydroxide will be. In general, anything below pH 10 is fine if it’s in contact with your skin for a short amount of time, although you’ll probably want to rinse your skin afterwards (soap is pH 9-10).
Speaking of soap…
Sodium hydroxide in soap
The issue of sodium hydroxide in soap is a bit like sodium hydroxide as a pH adjuster – in the final product, there isn’t much sodium hydroxide there. In soap, the sodium hydroxide reacts with a fat or oil, turning it into soap.
More information on soap chemistry and saponification: Make Your Own Soap! Part 1: The Chemistry Behind Soap Making
That means that once you have a soap, you shouldn’t have any sodium hydroxide left. Usually soap-makers actually play it safe and add extra fat or oil (“superfatting”) to make sure there’s no excess sodium hydroxide, so you don’t burn your skin off accidentally.
However, the ingredient lists in soaps are usually listed as the raw ingredients that were mixed together to make the soap, so sodium hydroxide will be at a disturbingly high position. But don’t worry – if the soap’s been made properly, the sodium hydroxide will be used up.
(Although whether you should be using soap at all is another question! Read more about choosing the right cleanser in this post and The Lab Muffin Guide to Basic Skincare).






so basically it all boils down to how the product is formulated if it’s within the PH that won’t distrupt the skin.
Yep, pretty much!
Really, you were being sarcastic? I ALMOST couldn´t tell…
Thanks for making chemistry easily understood. Much appreciated!
Thank you for the explanation, I was wondering about sodium hydroxide. I was using CeraVe PM Moisturizer with no reaction from my sensitive skin. Then they changed the formula, made it lighter and added sodium hydroxide. Now my skin breaks out in small bumps when I use it. I thought it was the sodium hydroxide, but I see that probably wasn’t the problem. Perhaps CeraVe (or L’Oreal who bought them out) added in more niacinimide, or some other ingredient my skin doesn’t like. Thanks again for the info, I appreciate it!
I’ve discovered your blog and channel a few months ago and for the first time in my life I am actually interested in and understanding a few things about chemistry, thanks for being able to explain things in such a clear way that even someone who is no expert in chemistry can understand!
And thanks for challenging myths on the web, because I have always been bothered by how many people keep alarming about certain ingredients but none of them can give plausible and scientific explanations on why those ingredients were bad! I am not the type of person who can simply believe in affirmations without logic, but I never had enough knowledge to go and read full scientific paper on parabens for instance.
Sorry for the long text, I just had to say how great is your work and I wish I could find someone who also have a more scientific approach on skincare in my mother tongue as well.
Thank you so much, I really appreciate you taking the time to let me know! 🙂
Not so much a chemistry question, but rather a biology one, I’m curious about the growing trend of topical probiotics in skincare (for instance, there is a moisturizer from Biossance). What is the evidence for skincare benefits, and what are the risks of disrupting your face’s baseline flora?
That’s a good question… and one that I’ve been meaning to look into! Unfortunately I don’t know enough about it to give a quick answer I’m confident about.
Lady, you are doing the Lord’s work.??❤
It does the beauty industry such a disservice when things like this are dumbed down. Kudos for being the voice of reason Michelle!
But how would I know the ph of my skin care product, like a toner?
Chemistry is real and applicable in every day life.I really appreciate for your great work as chemists.