Reminder: Only a few days left to enter my giveaway! Good luck everyone 🙂
This is the last of the Big 3. The other two were covered earlier: DBP – Toluene
Formaldehyde – form-AL-de-hide – MSDS
Other names: methanal. When mixed with water, formaldehyde forms methanediol, which is also known as formalin, methylene glycol or formaldehyde monohydrate.
Not to be confused with: formaldehyde resin
Where is it found? Formaldehyde, as it’s drawn above, is never found in cosmetics in any large amount (it’s a gas) – however, methanediol is found in nail hardening products, biology labs (as a preservative), and Brazilian keratin treatments. Formaldehyde-releasing chemicals, such as quaternium-15, imidazolidinyl urea, and diazolidinyl urea, are often found in tiny amounts as preservatives in all sorts of things (shampoo, perfume, body lotion…).
Is it dangerous?
This is a tricky one!
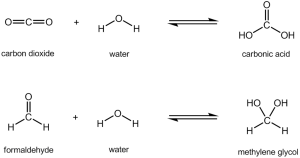
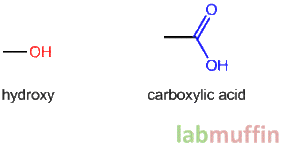
The story behind this nail polish ingredient is quite interesting! When you dissolve formaldehyde in water, it’s not just formaldehyde in water (unlike how sugar + water is just sugary water). It actually reacts to form methylene glycol, a different chemical:
The International Nomenclature of Cosmetic Ingredients (INCI), which tells cosmetic manufacturers what to write on their ingredient lists, used to get so that formalin is now correctly labelled as In 2008, the naming error was finally corrected. (The whole shebang is documented here.)
HOWEVER: the reaction between formaldehyde and water to form methylene glycol is like the reaction between carbon dioxide and water to give carbonic acid. It’s a special type of reaction known as a reversible reaction (you can tell from the double arrow). When a carbonated drink is in a bottle, it’s not fizzy yet… but when you release the pressure by opening the lid, you get heaps of carbon dioxide gas bubbles. Just like that reverse reaction, under the right conditions, the methylene glycol will turn back into formaldehyde and water. So even if formaldehyde itself isn’t in your nail hardening product, it’s possible that chemicals which release formaldehyde are!
Formaldehyde is, first off, an irritant, which means if you inhale too much it can cause get itchy, watery eyes, coughing, difficulty in breathing, and asthma attacks. It can also give you headaches.
It’s also a known human carcinogen – it’s been linked to nasopharyngeal and nasal sinus cancer, as well as leukemia.
There are legal limits in some countries on how much formaldehyde can be in your nail hardener, as well as special instructions on how to use them safely, which reduces the risk of side effects.
Lab Muffin’s verdict: Nasty! However, it’s one of the best nail hardeners. Take care when you use it, and try to use it in a well-ventilated place.







This is really interesting! I’m going to have to check for it…
Glad you liked it! 🙂
What’s formaldehyde resin & is it bad?
It’s part of the “polish” part of nail polish (i.e. the bit that stays on your nail when it’s dry) – from what I’ve read so far on formaldehyde resins they’re fine, but I’ll be covering that later, since it’s not in “4-free” polishes. I’ll also be looking at camphor, which is the 5th chemical taken out of “5-free” products 🙂
Super smart & interesting as always! Thank you 🙂
You’re too sweet 🙂 Thanks!
When I finally set up an area for my nail polish stuff it’s going to be near a window for ventilation…and the view 🙂
Yay! I’m always surprised by how much nail polish stinks, since in lab we use litres and litres of solvent but in or next to fume hoods pumping away. That said, I think I’ve developed a tolerance to most solvents now – it’s probably impossible to drug me with chloroform! Little-recognised side effect of being a lab muffin 🙂
Thanks for the explanation! So simple and easy to read, as always 🙂
🙂 Thanks for reading it!
Thank you for another great explanation 🙂
🙂 Glad you found it useful!
Aaah, I just found your blog!! Sooo cool… I have a masters in science so I actually do understand all the rxns and chemistry ‘jargon’ but you’re the first one who actually took effort to dig deeper and educate yourself and others about not the fact that these chemicals ARE bad, but WHY they are bad… Well done, girlfriend!!! You got a new follower 🙂
Thank you so much for your nice words! It means a lot to me 🙂 Hope my future posts don’t disappoint!