Shellac and other hybrid gel nails give long-lasting nail colour that can be soaked off with acetone. You simply paint your nails using special gel polishes, then “cure” each layer under UV light. What’s in those special polishes, and how does the UV light change that? Plastic – it’s fantastic To understand gel nails, first we need to understand plastic! Plastic is made up of long stringy molecules. Depending on how these molecules are arranged, and their exact composition, you can get different types of plastic. If they’re arranged neatly in lines, you end up with hard, rigid plastic, used for things like boxes and furniture. If they’re all jumbled up, you end up with soft, stretchy plastic, like in clingfilm and plastic bags. Polymerisation Plastic is made in a process called polymerisation – small molecules called monomers (mono = “one”) react in chain reactions to form long plastic molecules (polymers: poly = “many”). One polymer chain can be made up of thousands of monomers.
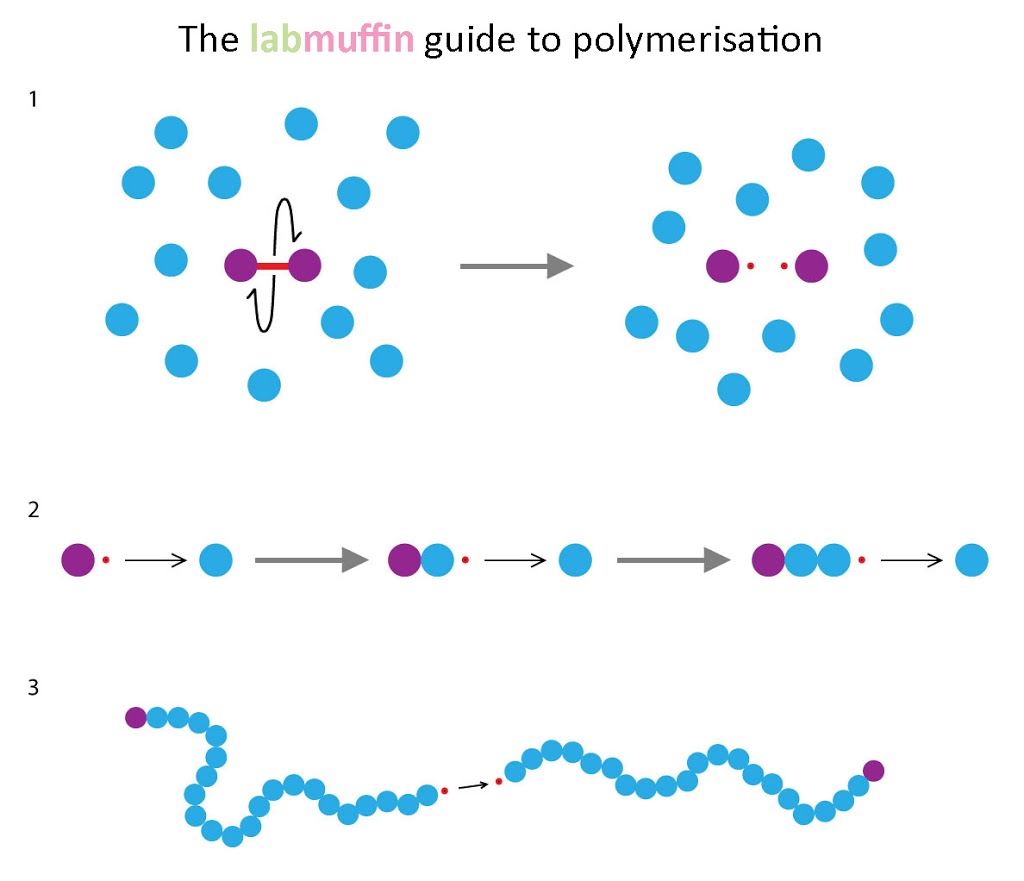
There are three major steps in polymerisation: 1. Initiation – free radicals (unpaired electrons – the red dots) are formed from bonds breaking symmetrically. 2. Chain elongation – the free radical attacks a monomer unit. The free radical is passed on, and the new radical can attack another monomer unit. This step repeats thousands of times. 3. Termination – The free radical attacks another free radical. Since a free radical is an unpaired electron, when two free radicals meet up they pair together and stop each other from reacting further. The final polymer chain is thousands of monomers long! The UV light helps the chemical bond break in such a way that free radicals form. Free radicals are very reactive, and when they attack the wrong chemicals (such as DNA, and bits of your cells), they can make your skin look old and cause cancer – that’s why using sunscreen is so important! In soak-off gels, these polymers can be dissolved using acetone. In older acrylic and gel systems, these polymers don’t dissolve, so you need to remove them physically using files or drills (eep). Hope that was interesting!






Wow I took a few material science engineering classes in uni, and this post totally takes me back. Thanks for going over this! You should explain how shatter nail polishes work one day:)
I’m planning to, but the info I’ve found so far on shatters tends to be a bit dodgy…
Ooh, what an interesting read!
I’m glad you found it interesting (and not super-boring)!
Oh man this brings me back to A-level chemistry days in college.
It’s weird when you find out that high school chemistry has real life applications you’re interested in!
Love your posts like this!
Eventually through improvement and scientific break through, would there be a way to set the polish using something other than UV light? Something simple like heat or coldness? Just a wonder of mine! Haha 🙂
Good question – some actually exist! In fact, in my research I’ve used a thermal initiator – that’s where heat is used to start the reaction instead of UV light. The one I used only needed to be heated up to 40 degrees Celcius (about warm shower temperature). The problem is that sometimes in transit, temperatures can get out of control, not to mention in hot cars, hot mail boxes etc., so you’d have to keep it in the fridge to make sure it didn’t all turn into one big plastic bottle-shaped chunk! On the other hand, UV light is relatively easy to block out – just have an opaque bottle. So there are definitely some big cons to simpler methods of curing gel polishes!
Oh right, i see thats really interesting! Thanks alot for that 🙂
I really enjoyed this post.
To speculate on how shatter works, we can all agree its cause the polish shrinks leaving gaps. If you’ve ever messed it up in terms of being too impatient and shoved shatter on not quite dry basecolour, its very likely it just gathered in a blob rather than cracking nicely. This would be cause even though it ‘stuck’ to the basecolour, the base wasn’t fixed itself so it was free to be moved as the shatter shrinked.
My guess is that the formula is almost like glue dissolved in a medium that evaporates. As it dries, the sticky particles come together and reduce the volume/area the polish occupies. Since these sticky particles also stick to the basecolour, there is some conflict of forces, some stick a bit more to the base, some stick to each other therefore don’t keep stuck to the base as they dry and thus create the cracks as they move about.
This isn’t taken from any scientific source, its just what I think happens judging from observations. I’m not a physicist, but did a bit of chemistry, so this might not be 100% accurate, however it makes a lot of sense to me. Looking forward to what you may find out.
This is an amazing physical process, who doesn’t love to see it dry?! 😀
Thanks for the post. I get here Very helpful information. Excellent blog right here! I was just searching for this information for a while .There is obviously a lot to know about this. I think you made some good points in Features also. I have been using latest collection of gel polish kit and I really satisfied.
Cindy
Phenomenal information, you explained everything quite scientifically but also in layman’s terms. Great job, loved this article!!