Hair is very important – it’s an easy body part to change, and can dramatically alter your appearance, but if you wake up with bad hair, your day is automatically terrible.
Luckily, it’s also very interesting, scientifically speaking, since we throw a lot of chemicals at it every day. Today’s post will be on electrostatic forces though – tiny changes which affect how your hair behaves. But first off, we have to have a little talk about…
Tiny positives and negatives
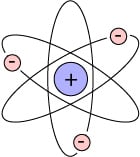
This is an atom – you might remember this diagram from high school science, or everywhere in pop culture:
The middle is positively charged, and the outer balls are negatively charged electrons. (For the sticklers in the audience, I realise I didn’t balance the charges!) Everything is made of atoms – solids, liquids and gases, including your hair.
As you might expect, the negative electrons on the outside are relatively easy to remove from atoms and add back, and in fact, that’s the basis of all chemical reactions, from fires burning to digestion to photosynthesis.
How to stick stuff to your hair
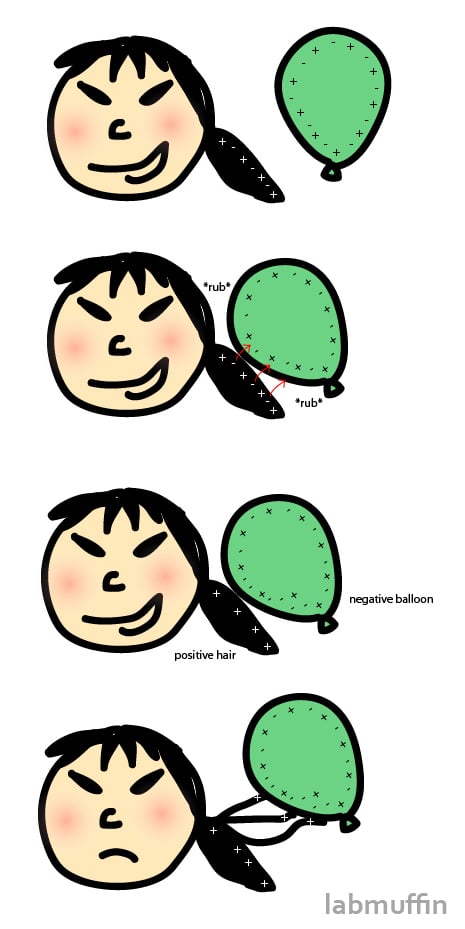
Hair static though, isn’t quite a chemical reaction – some electrons can just rub off from one material to another. For example, if you rub a balloon on your hair, some negative electrons come off your hair and cling to the balloon. This makes your hair positively charged, and the balloon negatively charged:
As you’ve heard before, “opposites attract” – this is exactly what happens! The positive hair and the negative balloon stick together, which means you can torture people like this:
 |
| from http://scienceblogs.com/startswithabang |
There’s actually a rough chart of what materials like to be positive and what materials like to be negative, called the triboelectric series. Here are some of the things on the list:
Items higher on the list tend to lose negative charges to things below them. So if you brush your hair with a plastic brush, chances are you’re charging up your hair to be positive. If the wind (air) is whipping your hair back and forth, your hair is also being charged up (to be negative).
Just like we discussed earlier, opposite attract… but things that are alike repel each other. So if all your hair is charged positive, the individual strands of hair will all repel each other, which makes it poofy and frizzy!
All this charge talk has implications for how hair conditioners work, which is also very interesting, but a topic for a post on its own…





So… I shouldn’t be using a plastic hairbrush to brush my hair?
Only if static is a problem for you! Static tends to affect fine hair, especially in short hairstyles, so it shouldn’t affect your hair too much.
Great post! I have really learned a lot here. Never knew this theory. So this is why we have frizzy hair. Now I know what will I use on my hair.
wow this is so interesting, i never know they was why hair gets frizzy!
My hair is fine, and it loves to frizz. Using an ionic hair dryer does make a difference! Not a cure, though…
nice faces even very interesting. research paper writing i think so helpful and thank you assistant about it.